Hydrogen cyanide on:
[Wikipedia]
[Google]
[Amazon]
Hydrogen cyanide, sometimes called prussic acid, is a
 Hydrogen cyanide was first isolated from a blue pigment ( Prussian blue) which had been known since 1706, but whose structure was unknown. It is now known to be a
Hydrogen cyanide was first isolated from a blue pigment ( Prussian blue) which had been known since 1706, but whose structure was unknown. It is now known to be a
. Cyanidecode.org. Retrieved on 2012-06-02. A hydrogen cyanide concentration of 2000 ppm (about 2380 mg/m3) will kill a human in about one minute. The toxic effect is caused by the action of the cyanide ion, which halts
Cyanure d'hydrogène et solutions aqueuses
. ''Fiche toxicologique n° 4'', Paris:INRS, 5pp. (PDF file, ''in French'')
( CICAD 61)
National Pollutant Inventory: Cyanide compounds fact sheetDepartment of health reviewDensity of Hydrogen Cyanide gas
{{DEFAULTSORT:Hydrogen Cyanide Blood agents Cyanides Fumigants Hydrogen compounds Inorganic compounds Gaseous signaling molecules Soviet chemical weapons program
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula HCN and structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such a ...
. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor
Precursor or Precursors may refer to:
* Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of u ...
to many chemical compounds ranging from polymers
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature.
Structure and general properties
Hydrogen cyanide is a linear molecule, with atriple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
between carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
. The tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hy ...
of HCN is HNC, hydrogen isocyanide.
Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would b ...
to give the cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
anion, CN−. A solution of hydrogen cyanide in water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, represented as HCN, is called ''hydrocyanic acid''. The salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
s of the cyanide anion are known as cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
s.
HCN has a faint bitter almond–like odor that some people are unable to detect owing to a recessive gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
tic trait. The volatile compound has been used as inhalation rodenticide and human poison, as well as for killing whales. Cyanide ions interfere with iron-containing respiratory enzymes.
Chemical properties
Hydrogen cyanide will react withalkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
s under catalysis of nickel complexes. This reaction is called hydrocyanation.
:RCH=CH2 + HCN → RCH2-CH2-CN
Four molecules of HCN will tetramerize into diaminomaleonitrile, which can be converted to various purines.
History of discovery
coordination polymer
A coordination polymer is an inorganic or organometallic polymer structure containing metal cation centers linked by ligands. More formally a coordination polymer is a coordination compound with repeating coordination entities extending in 1, 2 ...
with a complex structure and an empirical formula of hydrated ferric ferrocyanide. In 1752, the French chemist Pierre Macquer
Pierre-Joseph Macquer (9 October 1718 – 15 February 1784) was an influential French chemist.
He is known for his ''Dictionnaire de chymie'' (1766). He was also involved in practical applications, to medicine and industry, such as the French de ...
made the important step of showing that Prussian blue could be converted to an iron oxide plus a volatile component and that these could be used to reconstitute it. The new component was what is now known as hydrogen cyanide. Following Macquer's lead, it was first prepared from Prussian blue by the Swedish chemist Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
in 1782, and was eventually given the German name ''Blausäure'' (''lit''. "Blue acid") because of its acidic nature in water and its derivation from Prussian blue. In English, it became known popularly as ''prussic acid.''
In 1787, the French chemist Claude Louis Berthollet showed that prussic acid did not contain oxygen, an important contribution to acid theory, which had hitherto postulated that acids must contain oxygen (hence the name of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
itself, which is derived from Greek elements that mean "acid-former" and are likewise calque
In linguistics, a calque () or loan translation is a word or phrase borrowed from another language by literal word-for-word or root-for-root translation. When used as a verb, "to calque" means to borrow a word or phrase from another language ...
d into German as ''Sauerstoff''). In 1811, Joseph Louis Gay-Lussac prepared pure, liquified hydrogen cyanide. In 1815, Gay-Lussac deduced Prussic acid's chemical formula. The radical ''cyanide'' in hydrogen cyanide was given its name from cyan, not only an English word for a shade of blue but the Greek word for blue ( grc, κύανος), again owing to its derivation from Prussian blue.
Production and synthesis
Hydrogen cyanide forms in at least limited amounts from many combinations of hydrogen, carbon, andammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
. Hydrogen cyanide is currently produced in great quantities by several processes, as well as being a recovered waste product from the manufacture of acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecula ...
. In 2006, between 500 million and 1 billion pounds (between 230,000 and 450,000 t) were produced in the US.
The most important process is the Andrussow oxidation invented by Leonid Andrussow at IG Farben in which methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane ...
and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
react in the presence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
at about over a platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
catalyst:
:2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
The energy needed for the reaction is provided by the partial oxidation of methane and ammonia.
Of lesser importance is the Degussa process (BMA process The BMA process or Degussa process is a chemical process developed by the German chemical company Degussa for the production of hydrogen cyanide from methane and ammonia in presence of a platinum catalyst. Hydrogen cyanide is used in the chemical in ...
) in which no oxygen is added and the energy must be transferred indirectly through the reactor wall:
:CH4 + NH3 → HCN + 3H2
This reaction is akin to steam reforming, the reaction of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane ...
and water to give carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
.
In the Shawinigan Process, hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
, e.g. propane, are reacted with ammonia.
In the laboratory, small amounts of HCN are produced by the addition of acids to cyanide salts of alkali metals:
:H+ + NaCN → HCN + Na+
This reaction is sometimes the basis of accidental poisonings because the acid converts a nonvolatile cyanide salt into the gaseous HCN.
Historical methods of production
The large demand for cyanides for mining operations in the 1890s was met by George Thomas Beilby, who patented a method to produce hydrogen cyanide by passingammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
over glowing coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when ...
in 1892. This method was used until Hamilton Castner
Hamilton Young Castner (September 11, 1858 – October 11, 1899) was an American industrial chemist.
Biography
He was born in Brooklyn, New York and educated at the Brooklyn Polytechnic Institute, then at the Columbia University School of Mine ...
in 1894 developed a synthesis starting from coal, ammonia, and sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
yielding sodium cyanide, which reacts with acid to form gaseous HCN.
Applications
HCN is the precursor to sodium cyanide and potassium cyanide, which are used mainly ingold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
and silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
mining and for the electroplating of those metals. Via the intermediacy of cyanohydrin
In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is , where R is H, alkyl, or aryl. Cyanohyd ...
s, a variety of useful organic compounds are prepared from HCN including the monomer
In chemistry, a monomer ( ; '' mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
methyl methacrylate, from acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
, the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
methionine, via the Strecker synthesis, and the chelating agents EDTA and NTA. Via the hydrocyanation process, HCN is added to butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two v ...
to give adiponitrile, a precursor to Nylon-6,6.
HCN is used globally as a fumigant against many species of pest insect that infest food production facilities. Both its efficacy and method of application lead to very small amounts of the fumigant being used compared to other toxic substances used for the same purpose. Using HCN as a fumigant also has minimal environmental impact, compared to similar structural fumigant molecules such as sulfuryl fluoride, and methyl bromide.
Occurrence
HCN is obtainable fromfruit
In botany, a fruit is the seed-bearing structure in flowering plants that is formed from the ovary after flowering.
Fruits are the means by which flowering plants (also known as angiosperms) disseminate their seeds. Edible fruits in partic ...
s that have a pit, such as cherries, apricots, apple
An apple is an edible fruit produced by an apple tree (''Malus domestica''). Apple trees are cultivated worldwide and are the most widely grown species in the genus '' Malus''. The tree originated in Central Asia, where its wild ancest ...
s, and bitter almonds, from which almond oil and flavoring are made. Many of these pits contain small amounts of cyanohydrin
In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is , where R is H, alkyl, or aryl. Cyanohyd ...
s such as mandelonitrile
In organic chemistry, mandelonitrile is the nitrile of mandelic acid, or the cyanohydrin derivative of benzaldehyde. Small amounts of mandelonitrile occur in the pits of some fruits.
Occurrence
Mandelonitrile is the aglycone part of the cyano ...
and amygdalin, which slowly release hydrogen cyanide. One hundred grams of crushed apple seeds can yield about 70 mg of HCN. So-called "bitter" roots of the cassava
''Manihot esculenta'', commonly called cassava (), manioc, or yuca (among numerous regional names), is a woody shrub of the spurge family, Euphorbiaceae, native to South America. Although a perennial plant, cassava is extensively cultivated ...
plant may contain up to 1 gram of HCN per kilogram. Some millipedes, such as '' Harpaphe haydeniana'', '' Desmoxytes purpurosea'', and ''Apheloria
''Apheloria'' is a genus of flat-backed millipedes in the family Xystodesmidae, occurring in the central and southeastern United States, and ranging as far north as southern Quebec, Canada.Hoffman, Richard L. 1999. Checklist of the millipeds of ...
'' release hydrogen cyanide as a defense mechanism, as do certain insects, such as burnet moths and the larvae of '' Paropsisterna eucalyptus''. Hydrogen cyanide is contained in the exhaust of vehicles, and in smoke from burning nitrogen-containing plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adapta ...
s.
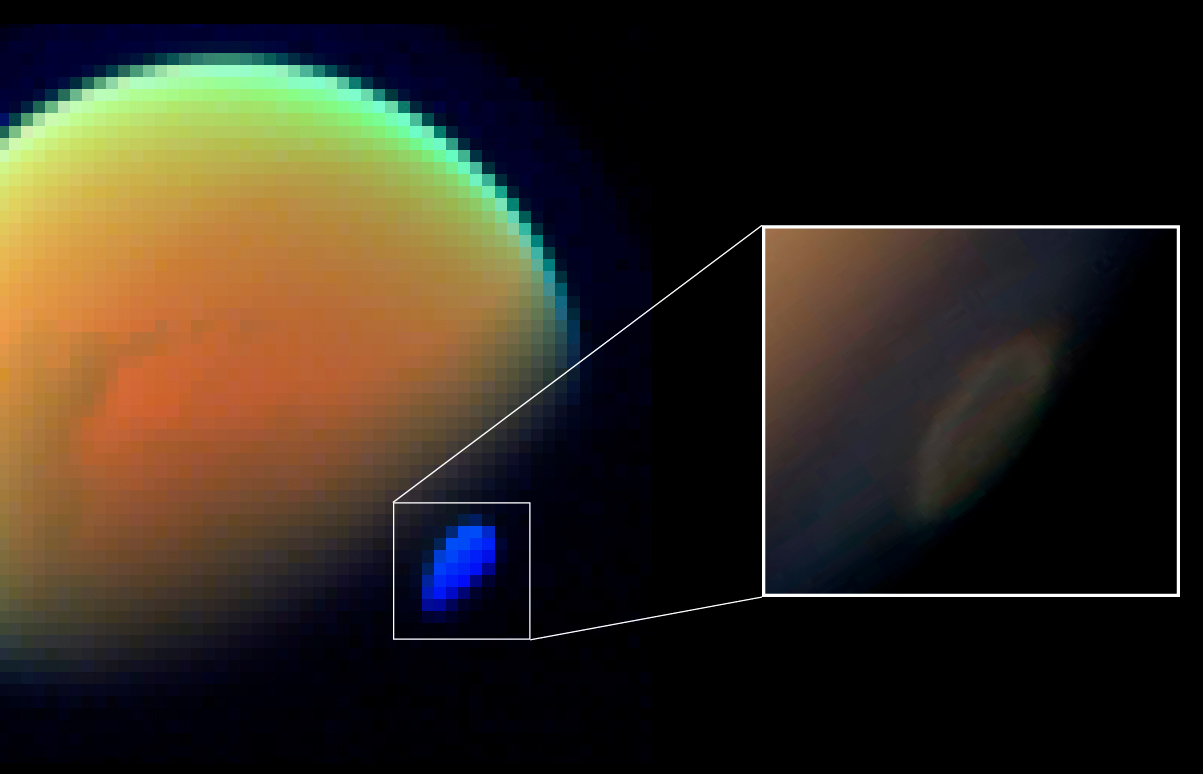
HCN on Titan
HCN has been measured in Titan's atmosphere by four instruments on theCassini space probe Cassini may refer to:
People
* Cassini (surname)
* Oleg Cassini (1913-2006), American fashion designer
:Cassini family:
* Giovanni Domenico Cassini (1625–1712), Italian mathematician, astronomer, engineer, and astrologer
* Jacques Cassini (167 ...
, one instrument on Voyager
Voyager may refer to:
Computing and communications
* LG Voyager, a mobile phone model manufactured by LG Electronics
* NCR Voyager, a computer platform produced by NCR Corporation
* Voyager (computer worm), a computer worm affecting Oracle ...
, and one instrument on Earth. One of these measurements was ''in situ'', where the Cassini spacecraft dipped between above Titan's surface to collect atmospheric gas for mass spectrometry analysis. HCN initially forms in Titan's atmosphere through the reaction of photochemically produced methane and nitrogen radicals which proceed through the H2CN intermediate, e.g., (CH3 + N → H2CN + H → HCN + H2). Ultraviolet radiation breaks HCN up into CN + H; however, CN is efficiently recycled back into HCN via the reaction CN + CH4 → HCN + CH3.
HCN on the young Earth
It has been postulated that carbon from a cascade of asteroids (known as the Late Heavy Bombardment), resulting from interaction of Jupiter and Saturn, blasted the surface of young Earth and reacted with nitrogen in Earth's atmosphere to form HCN.HCN in mammals
Some authors have shown thatneuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa ...
s can produce hydrogen cyanide upon activation of their opioid
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use ...
receptors by endogenous or exogenous opioids. They have also shown that neuronal production of HCN activates NMDA receptors and plays a role in signal transduction between neuronal cells ( neurotransmission). Moreover, increased endogenous neuronal HCN production under opioids was seemingly needed for adequate opioid analgesia, as analgesic action of opioids was attenuated by HCN scavengers. They considered endogenous HCN to be a neuromodulator.
It has also been shown that, while stimulating muscarinic cholinergic receptors in cultured pheochromocytoma
Pheochromocytoma (PHEO or PCC) is a rare tumor of the adrenal medulla composed of chromaffin cells, also known as pheochromocytes. When a tumor composed of the same cells as a pheochromocytoma develops outside the adrenal gland, it is referred t ...
cells ''increases'' HCN production, in a living organism (''in vivo'') muscarinic cholinergic stimulation actually ''decreases'' HCN production.
Leukocytes generate HCN during phagocytosis, and can kill bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
, fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately fr ...
, and other pathogens by generating several different toxic chemicals, one of which is hydrogen cyanide.
The vasodilatation caused by sodium nitroprusside has been shown to be mediated not only by NO generation, but also by endogenous cyanide generation, which adds not only toxicity, but also some additional antihypertensive efficacy compared to nitroglycerine and other non-cyanogenic nitrates which do not cause blood cyanide levels to rise.
HCN is a constituent of tobacco smoke.
HCN and the origin of life
Hydrogen cyanide has been discussed as a precursor to amino acids and nucleic acids, and is proposed to have played a part in the origin of life. Although the relationship of these chemical reactions to the origin of life theory remains speculative, studies in this area have led to discoveries of new pathways to organic compounds derived from the condensation of HCN (e.g.Adenine
Adenine () ( symbol A or Ade) is a nucleobase (a purine derivative). It is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The three others are guanine, cytosine and thymine. Its deriv ...
).
HCN in space
HCN has been detected in the interstellar medium and in the atmospheres of carbon stars. Since then, extensive studies have probed formation and destruction pathways of HCN in various environments and examined its use as a tracer for a variety of astronomical species and processes. HCN can be observed from ground-basedtelescope
A telescope is a device used to observe distant objects by their emission, absorption, or reflection of electromagnetic radiation. Originally meaning only an optical instrument using lenses, curved mirrors, or a combination of both to obse ...
s through a number of atmospheric windows. The J=1→0, J=3→2, J= 4→3, and J=10→9 pure rotational transitions have all been observed.
HCN is formed in interstellar clouds through one of two major pathways: via a neutral-neutral reaction (CH2 + N → HCN + H) and via dissociative recombination
Dissociative recombination is a chemical process where a positive polyatomic ion recombines with an electron, and as a result, the neutral molecule dissociates. This reaction is important for extraterrestrial and atmospheric chemistry. On Earth, ...
(HCNH+ + e− → HCN + H). The dissociative recombination pathway is dominant by 30%; however, the HCNH+ must be in its linear form. Dissociative recombination with its structural isomer, H2NC+, exclusively produces hydrogen isocyanide (HNC).
HCN is destroyed in interstellar clouds through a number of mechanisms depending on the location in the cloud. In photon-dominated region
In astrophysics, photodissociation regions (or photon-dominated regions, PDRs) are predominantly neutral regions of the interstellar medium in which far ultraviolet photons strongly influence the gas chemistry and act as the most important source ...
s (PDRs), photodissociation dominates, producing CN (HCN + ν → CN + H). At further depths, photodissociation by cosmic rays dominate, producing CN (HCN + cr → CN + H). In the dark core, two competing mechanisms destroy it, forming HCN+ and HCNH+ (HCN + H+ → HCN+ + H; HCN + HCO+ → HCNH+ + CO). The reaction with HCO+ dominates by a factor of ~3.5. HCN has been used to analyze a variety of species and processes in the interstellar medium. It has been suggested as a tracer for dense molecular gas and as a tracer of stellar inflow in high-mass star-forming regions. Further, the HNC/HCN ratio has been shown to be an excellent method for distinguishing between PDRs and X-ray-dominated regions (XDRs).
On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust
Dust is made of fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian process), volcanic eruptions, and pollution. Dust in ...
inside the comae of comet
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena ...
s C/2012 F6 (Lemmon) and C/2012 S1 (ISON).
In February 2016, it was announced that traces of hydrogen cyanide were found in the atmosphere of the hot Super-Earth 55 Cancri e with NASA's Hubble Space Telescope
The Hubble Space Telescope (often referred to as HST or Hubble) is a space telescope that was launched into low Earth orbit in 1990 and remains in operation. It was not the first space telescope, but it is one of the largest and most vers ...
.
As a poison and chemical weapon
InWorld War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was List of wars and anthropogenic disasters by death toll, one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, ...
, hydrogen cyanide was used by the French from 1916 as a chemical weapon against the Central Powers
The Central Powers, also known as the Central Empires,german: Mittelmächte; hu, Központi hatalmak; tr, İttifak Devletleri / ; bg, Централни сили, translit=Tsentralni sili was one of the two main coalitions that fought in W ...
, and by the United States and Italy
Italy ( it, Italia ), officially the Italian Republic, ) or the Republic of Italy, is a country in Southern Europe. It is located in the middle of the Mediterranean Sea, and its territory largely coincides with the homonymous geographical ...
in 1918. It was not found to be effective enough due to weather conditions. The gas is lighter than air and rapidly disperses up into the atmosphere. Rapid dilution made its use in the field impractical. In contrast, denser agents such as phosgene or chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
tended to remain at ground level and sank into the trenches of the Western Front's battlefields. Compared to such agents, hydrogen cyanide had to be present in higher concentrations in order to be fatal.
A hydrogen cyanide concentration of 100–200 ppm in breathing air will kill a human within 10 to 60 minutes.Environmental and Health Effects. Cyanidecode.org. Retrieved on 2012-06-02. A hydrogen cyanide concentration of 2000 ppm (about 2380 mg/m3) will kill a human in about one minute. The toxic effect is caused by the action of the cyanide ion, which halts
cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
. It acts as a non-competitive inhibitor for an enzyme in mitochondria called cytochrome c oxidase. As such, hydrogen cyanide is commonly listed among chemical weapons as a blood agent
A blood agent is a toxic chemical agent that affects the body by being absorbed into the blood. Blood agents are fast-acting, potentially lethal poisons that typically manifest at room temperature as volatile colorless gases with a faint odor. T ...
.
The Chemical Weapons Convention lists it under Schedule 3 as a potential weapon which has large-scale industrial uses. Signatory countries must declare manufacturing plants that produce more than 30 metric tons per year, and allow inspection by the Organisation for the Prohibition of Chemical Weapons
The Organisation for the Prohibition of Chemical Weapons (OPCW) is an intergovernmental organisation and the implementing body for the Chemical Weapons Convention (CWC), which entered into force on 29 April 1997. The OPCW, with its 193 member ...
.
Perhaps its most infamous use is (German: ''Cyclone B'', with the B standing for – prussic acid; also, to distinguish it from an earlier product later known as Zyklon A), used in Nazi German extermination camps during World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the World War II by country, vast majority of the world's countries—including all of the great power ...
to kill ''en masse'' as part of their Final Solution genocide program. Hydrogen cyanide was also used in the camps for delousing clothing in attempts to eradicate diseases carried by lice and other parasites. One of the original Czech producers continued making Zyklon B under the trademark "Uragan D2" until around 2015.
During World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the World War II by country, vast majority of the world's countries—including all of the great power ...
, the US considered using it, along with cyanogen chloride, as part of Operation Downfall, the planned invasion of Japan, but President Harry Truman decided against it, instead using the atomic bombs developed by the secret Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
.
Hydrogen cyanide was also the agent employed in judicial execution in some U.S. states, where it was produced during the execution by the action of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
on sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
or potassium cyanide.
Under the name ''prussic acid'', HCN has been used as a killing agent in whaling
Whaling is the process of hunting of whales for their usable products such as meat and blubber, which can be turned into a type of oil that became increasingly important in the Industrial Revolution.
It was practiced as an organized industr ...
harpoons, although it proved quite dangerous to the crew deploying it, and it was quickly abandoned. From the middle of the 18th century it was used in a number of poisoning murders and suicides.
Hydrogen cyanide gas in air is explosive at concentrations above 5.6%. This concentration is far above a toxic level.
References
External links
*Institut national de recherche et de sécurité (1997).Cyanure d'hydrogène et solutions aqueuses
. ''Fiche toxicologique n° 4'', Paris:INRS, 5pp. (PDF file, ''in French'')
( CICAD 61)
National Pollutant Inventory: Cyanide compounds fact sheet
{{DEFAULTSORT:Hydrogen Cyanide Blood agents Cyanides Fumigants Hydrogen compounds Inorganic compounds Gaseous signaling molecules Soviet chemical weapons program