Heat of fusion on:
[Wikipedia]
[Google]
[Amazon]
 In
In
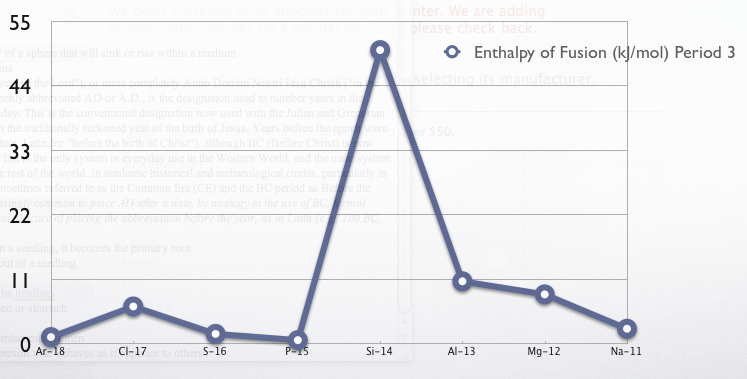
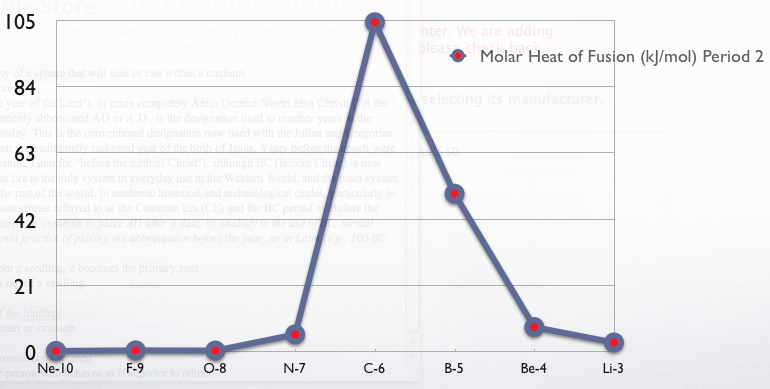 {, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
,
{, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
,
Thermal Energy Storage: Systems and Applications
page 155 , - , stearic acid , 47.54 , 198.91 , - , gallium , 19.2 , 80.4 , - ,
thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of th ...
, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
resulting from providing energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of hea ...
, typically heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ...
, to a specific quantity of the substance to change its state
State may refer to:
Arts, entertainment, and media Literature
* ''State Magazine'', a monthly magazine published by the U.S. Department of State
* ''The State'' (newspaper), a daily newspaper in Columbia, South Carolina, United States
* ''Our S ...
from a solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural ...
to a liquid, at constant pressure.
It is the amount of energy required to convert one mole of solid into liquid For example, when melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
1 kg of ice (at 0 °C under a wide range of pressures), 333.55 kJ of energy is absorbed with no temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
change. The heat of solidification (when a substance changes from liquid to solid) is equal and opposite.
This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states o ...
occurs is the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
or the freezing point, according to context. By convention, the pressure is assumed to be unless otherwise specified.
Overview
The 'enthalpy' of fusion is alatent heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition.
Latent heat can be underst ...
, because, while melting, the heat energy needed to change the substance from solid to liquid at atmospheric pressure is latent heat of fusion, as the temperature remains constant during the process. The latent heat of fusion is the enthalpy change of any amount of substance when it melts. When the heat of fusion is referenced to a unit of mass, it is usually called the specific heat of fusion, while the molar heat of fusion refers to the enthalpy change per amount of substance in mole
Mole (or Molé) may refer to:
Animals
* Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America
* Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...
s.
The liquid phase has a higher internal energy than the solid phase. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes, because the molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s in the liquid experience weaker intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. ...
s and so have a higher potential energy (a kind of bond-dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical ...
for intermolecular forces).
When liquid water is cooled, its temperature falls steadily until it drops just below the line of freezing point at 0 °C. The temperature then remains constant at the freezing point while the water crystallizes. Once the water is completely frozen, its temperature continues to fall.
The enthalpy of fusion is almost always a positive quantity; helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
is the only known exception. Helium-3 has a negative enthalpy of fusion at temperatures below 0.3 K. Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consis ...
also has a very slightly negative enthalpy of fusion below . This means that, at appropriate constant pressures, these substances freeze with the addition of heat. In the case of 4He, this pressure range is between 24.992 and .
 {, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
,
{, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
, water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, 79.72
, 333.55
, -
, methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
, 13.96
, 58.99
, -
, propane
, 19.11
, 79.96
, -
, glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
, 47.95
, 200.62
, -
, formic acid
, 66.05
, 276.35
, -
, acetic acid
, 45.90
, 192.09
, -
, acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
, 23.42
, 97.99
, -
, benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, 30.45
, 127.40
, -
, myristic acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nut ...
, 47.49
, 198.70
, -
, palmitic acid
Palmitic acid (hexadecanoic acid in IUPAC nomenclature) is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms.Gunstone, F. D., John L. Harwood, and Albert J. Dijkstra. The L ...
, 39.18
, 163.93
, -
, sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
,
, 264–289Ibrahim Dincer and Marc A. RosenThermal Energy Storage: Systems and Applications
page 155 , - , stearic acid , 47.54 , 198.91 , - , gallium , 19.2 , 80.4 , - ,
paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to ...
(C25H52)
, 47.8–52.6
, 200–220
These values are mostly from the CRC ''Handbook of Chemistry and Physics'', 62nd edition. The conversion between cal/g and J/g in the above table uses the thermochemical calorie (calth) = 4.184 joules rather than the International Steam Table calorie (calINT) = 4.1868 joules.
Examples
Solubility prediction
The heat of fusion can also be used to predictsolubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
for solids in liquids. Provided an ideal solution is obtained the mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ex ...
of solute at saturation is a function of the heat of fusion, the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
of the solid and the temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
of the solution:
:
Here, is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
. For example, the solubility of paracetamol in water at 298 K is predicted to be:
:
Since the molar mass of water and paracetamol are and and the density of the solution is , an estimate of the solubility in grams per liter is:
:
which is a deviation from the real solubility (240 g/L) of 11%. This error can be reduced when an additional heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
parameter is taken into account.''Measurement and Prediction of Solubility of Paracetamol in Water-Isopropanol Solution. Part 2. Prediction'' H. Hojjati and S. Rohani Org. Process Res. Dev.; 2006; 10(6) pp 1110–1118; (Article)
Proof
At equilibrium thechemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species ...
s for the pure solvent and pure solid are identical:
:
or
:
with the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and the temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
.
Rearranging gives:
:
and since
:
the heat of fusion being the difference in chemical potential between the pure liquid and the pure solid, it follows that
:
Application of the Gibbs–Helmholtz equation
The Gibbs–Helmholtz equation is a thermodynamic equation used for calculating changes in the Gibbs free energy of a system as a function of temperature. It was originally presented in an 1882 paper entitled " Die Thermodynamik chemischer Vorgang ...
:
:
ultimately gives:
:
or:
:
and with integration:
:
the end result is obtained:
:
See also
* Heat of vaporization *Heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
* Thermodynamic databases for pure substances
* Joback method (Estimation of the heat of fusion from molecular structure)
* Latent heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition.
Latent heat can be underst ...
* Lattice energy
In chemistry, the lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed to initially be in the gaseous state. It is a measure of the cohesive forces that bin ...
* Heat of dilution
In thermochemistry, the heat of dilution, or enthalpy of dilution, refers to the enthalpy change associated with the dilution process of a component in a solution at a constant pressure. If the initial state of the component is a pure liquid ( ...
Notes
References
* * {{DEFAULTSORT:Enthalpy Of Fusion Thermodynamic properties Enthalpy