HCo(CO)4 on:
[Wikipedia]
[Google]
[Amazon]
Cobalt tetracarbonyl hydride is an
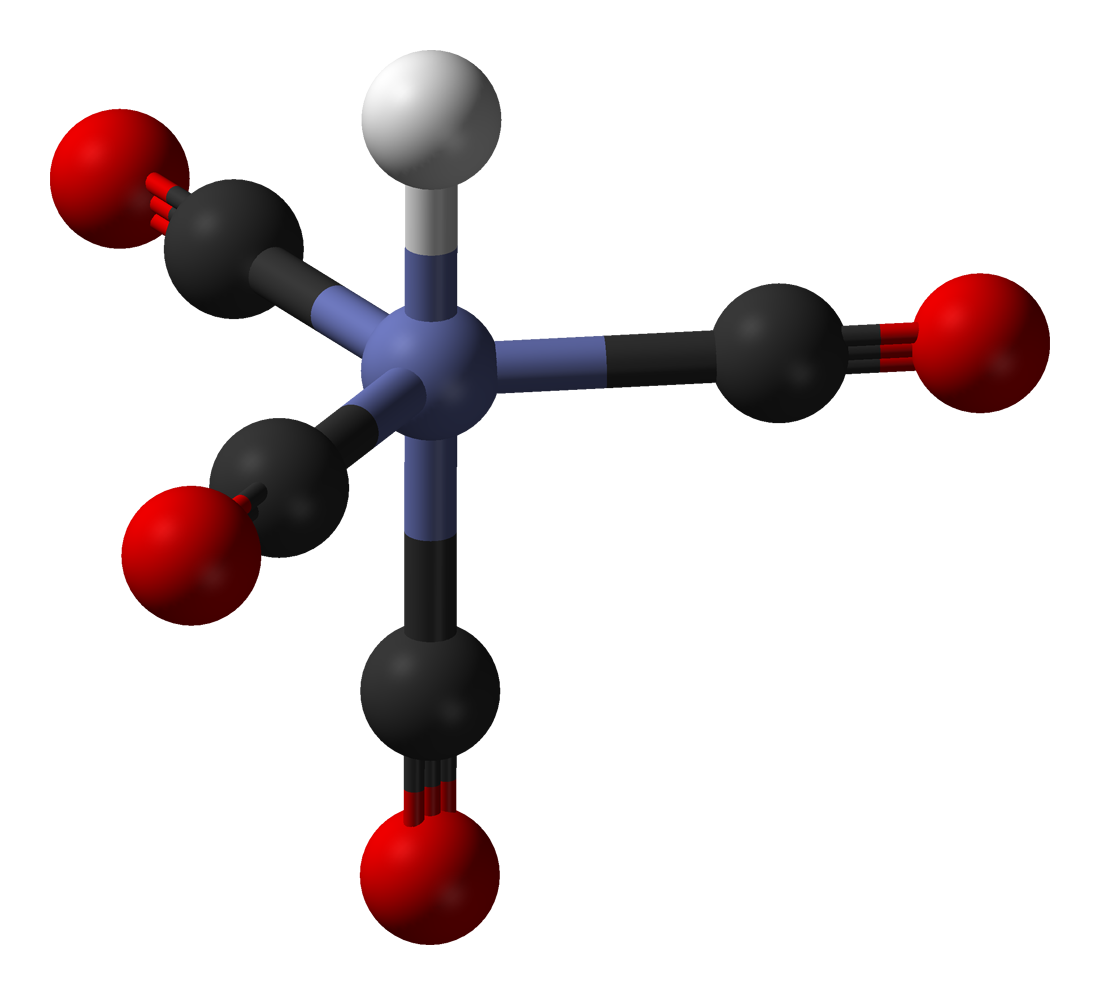 HCo(CO)4 adopts trigonal bipyramidal structure with the equatorial CO ligands slightly bent out of the equatorial plane. The hydride
HCo(CO)4 adopts trigonal bipyramidal structure with the equatorial CO ligands slightly bent out of the equatorial plane. The hydride
organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
with the formula H Co(CO)4. It is a volatile, yellow liquid that forms a colorless vapor and has an intolerable odor. The compound readily decomposes upon melt and ''in absentia'' of high CO partial pressures forms Co2(CO)8. Despite operational challenges associated with its handling, the compound has received considerable attention for its ability to function as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
in hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
. In this respect, HCo(CO)4 and related derivatives have received significant academic interest for their ability to mediate a variety of carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbony ...
(introduction of CO into inorganic compounds
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemi ...
) reactions.
Structure and properties
 HCo(CO)4 adopts trigonal bipyramidal structure with the equatorial CO ligands slightly bent out of the equatorial plane. The hydride
HCo(CO)4 adopts trigonal bipyramidal structure with the equatorial CO ligands slightly bent out of the equatorial plane. The hydride ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
occupies one of the axial positions, thus the symmetry of the molecule is ''C''3''v''. The Co–CO and Co–H bond distances were determined by gas-phase electron diffraction to be 1.764 and 1.556 Å, respectively. Assuming the presence of a formal hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
ion, the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
in this compound is +1.
But unlike some other transition-metal hydrides complexes, HCo(CO)4 is highly acidic, with a p''K''a of 8.5. It readily undergoes substitution by tertiary phosphines and other Lewis-bases. For example, triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
gives HCo(CO)3PPh3 and HCo(CO)2(PPh3)2. These derivatives are more stable than HCo(CO)4 and are used industrially to improve catalyst selectivity in hydroformylation. These derivatives are generally less acidic than HCo(CO)4.
Preparation
Tetracarbonylhydrocobalt was first described by Hieber in the early 1930s. It was the second transition metal hydride to be discovered, after H2Fe(CO)4. It is prepared by reducing Co2(CO)8 with sodium amalgam or a similar reducing agent followed by acidification. :Co2(CO)8 + 2 Na → 2 NaCo(CO)4 :NaCo(CO)4 + H+ → HCo(CO)4 + Na+ Since HCo(CO)4 decomposes so readily, it is usually generated ''in situ'' byhydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
of Co2(CO)8.
:Co2(CO)8 + H2 2 HCo(CO)4
The thermodynamic parameters for the equilibrium reaction were determined by infrared spectroscopy to be Δ''H'' = 4.054 kcal mol−1, ΔS = −3.067 cal mol−1 K−1.
Applications
Tetracarbonylhydridocobalt was the first transition metal hydride to be used in industry. In 1953 evidence was disclosed that it is the active catalyst for the conversion of alkenes, CO, and H2 toaldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s, a process known as hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
(oxo reaction). Although the use of cobalt-based hydroformylation has since been largely superseded by rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
-based catalysts, the world output of C3–C18 aldehydes produced by tetracarbonylhydrocobalt catalysis is about 100,000 tons/year, roughly 2% of the total.
References
{{cobalt compounds Organocobalt compounds Metal hydrides Carbonyl complexes Substances discovered in the 1930s