Grignard reagents on:
[Wikipedia]
[Google]
[Amazon]
 A Grignard reagent or Grignard compound is a
A Grignard reagent or Grignard compound is a
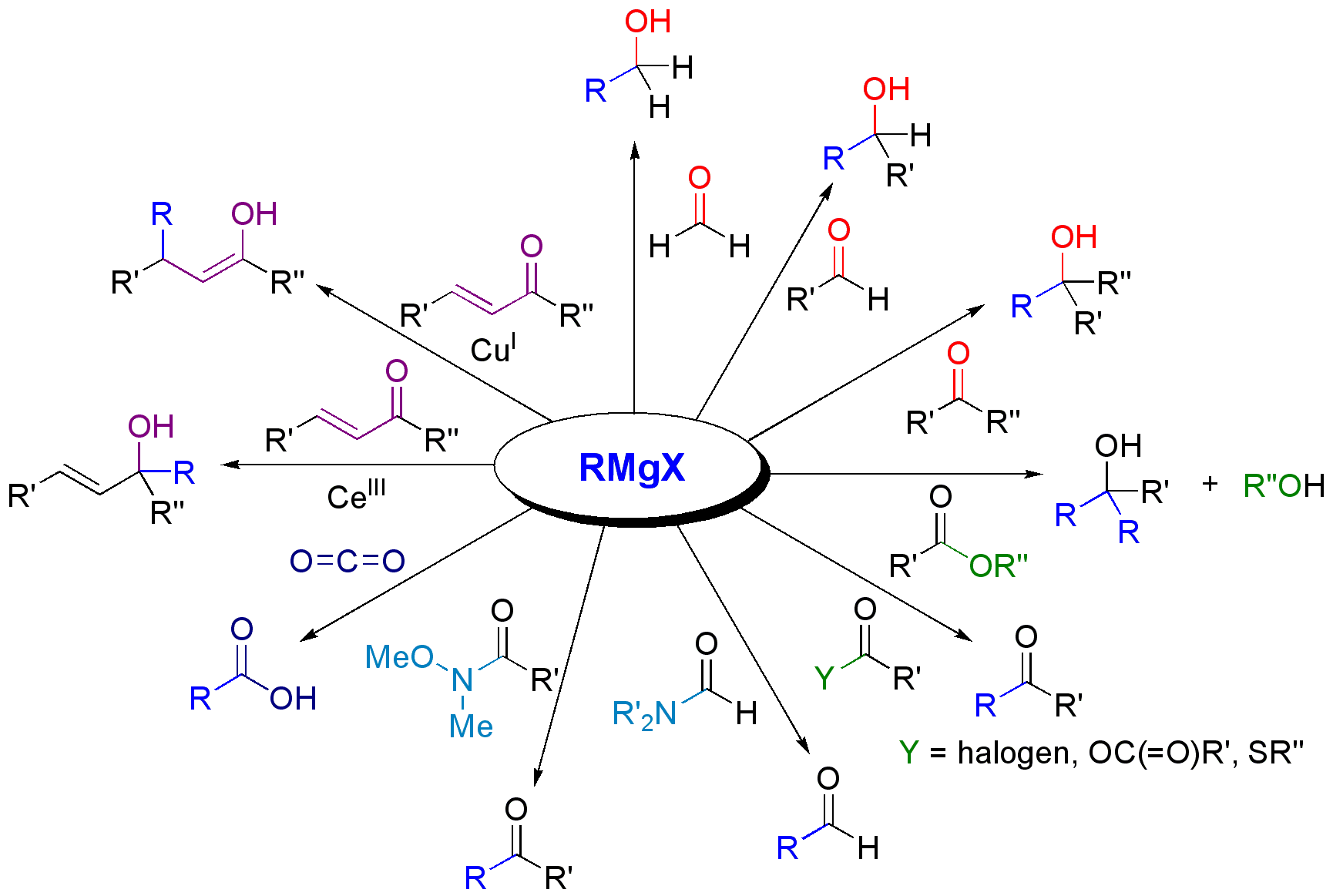 The most common application of Grignard reagents is the alkylation of aldehydes and ketones, i.e. ''the'' Grignard reaction:
The most common application of Grignard reagents is the alkylation of aldehydes and ketones, i.e. ''the'' Grignard reaction:
 Note that the
Note that the 

 For the coupling of aryl halides with aryl Grignard reagents,
For the coupling of aryl halides with aryl Grignard reagents,



Grignard reaction experiment 01.jpg, Magnesium turnings are placed in a flask.
Grignard reaction experiment 02.jpg, Tetrahydrofuran and a small piece of iodine are added.
Grignard reaction experiment 03.jpg, A solution of alkyl bromide is added while heating.
Grignard reaction experiment 04.jpg, After completion of the addition, the mixture is heated for a while.
Grignard reaction experiment 05.jpg, Formation of the Grignard reagent is complete. A small amount of magnesium still remains in the flask.
Grignard reaction experiment 06.jpg, The Grignard reagent thus prepared is cooled to before the addition of the carbonyl compound. The solution becomes cloudy as the Grignard reagent precipitates out.
Grignard reaction experiment 07.jpg, A solution of carbonyl compound is added to the Grignard reagent.
Grignard reaction experiment 08.jpg, The solution is warmed to room temperature. At this point the reaction is complete.
 A Grignard reagent or Grignard compound is a
A Grignard reagent or Grignard compound is a chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the general formula , where X is a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this grou ...
and R is an organic group, normally an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
. Two typical examples are methylmagnesium chloride
Methylmagnesium chloride is an organometallic compound with the general formula CH3MgCl. This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in ...
and phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is ...
. They are a subclass of the organomagnesium compounds.
Grignard compounds are popular reagents in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents.
Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
or tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom connected to the two ether oxygens by coordination bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal io ...
s.
The discovery of the Grignard reaction in 1900 was awarded with the Nobel prize in 1912. For more details on the history see Victor Grignard.
Synthesis
From Mg metal
Traditionally Grignard reagents are prepared by treating an organic halide (normally organobromine) with magnesium metal.Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again ...
s are required to stabilize the organomagnesium compound. Water and air, which rapidly destroy the reagent by protonolysis or oxidation, are excluded using air-free techniques. Although the reagents still need to be dry, ultrasound can allow Grignard reagents to form in wet solvents by activating the magnesium such that it consumes the water.
As is common for reactions involving solids and solution, the formation of Grignard reagents is often subject to an induction period. During this stage, the passivating oxide on the magnesium is removed. After this induction period, the reactions can be highly exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
. This exothermicity must be considered when a reaction is scaled-up from laboratory to production plant.
Most organohalides will work, but carbon-fluorine bonds are generally unreactive, except with specially activated magnesium (through Rieke metals).
Magnesium
Typically the reaction to form Grignard reagents involves the use of magnesium ribbon. All magnesium is coated with a passivating layer ofmagnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2� ...
, which inhibits reactions with the organic halide. Many methods have been developed to weaken this passivating layer, thereby exposing highly reactive magnesium to the organic halide. Mechanical methods include crushing of the Mg pieces in situ, rapid stirring, and sonication. Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
, methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one ...
, and 1,2-dibromoethane are common activating agents. The use of 1,2-dibromoethane is advantageous as its action can be monitored by the observation of bubbles of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
. Furthermore, the side-products are innocuous:
: Mg + BrC2H4Br → C2H4 + MgBr2
The amount of Mg consumed by these activating agents is usually insignificant. A small amount of mercuric chloride will amalgamate
Amalgamation is the process of combining or uniting multiple entities into one form.
Amalgamation, amalgam, and other derivatives may refer to:
Mathematics and science
* Amalgam (chemistry), the combination of mercury with another metal
**Pan ama ...
the surface of the metal, enhancing its reactivity. Addition of preformed Grignard reagent is often used as the initiator.
Specially activated magnesium, such as Rieke magnesium, circumvents this problem. The oxide layer can also be broken up using ultrasound, using a stirring rod to scratch the oxidized layer off, or by adding a few drops of iodine or 1,2-Diiodoethane. Another option is to use sublimed magnesium or magnesium anthracene
Magnesium anthracene is an organomagnesium compound that is almost invariably isolated as its adduct with three tetrahydrofuran (thf) ligands. With the formula Mg(C14H10)(thf)3, this air- and water-sensitive orange solid is obtained by heating a ...
.
Mechanism
In terms of mechanism, the reaction proceeds through single electron transfer: :R−X + Mg → R−X•− + Mg•+ :R−X•− → R• + X− :R• + Mg•+ → RMg+ :RMg+ + X− → RMgXMg transfer reaction (halogen–Mg exchange)
An alternative preparation of Grignard reagents involves transfer of Mg from a preformed Grignard reagent to an organic halide. Other organomagnesium reagents are used as well. This method offers the advantage that the Mg transfer tolerates many functional groups. An illustrative reaction involvesisopropylmagnesium chloride
Isopropylmagnesium chloride is an organometallic compound with the general formula (CH3)2HCMgCl. This highly flammable, colorless, and moisture sensitive material is the Grignard reagent derived from isopropyl chloride. It is commercially avail ...
and aryl bromide or iodides:
:''i''-PrMgCl + ArCl → ''i''-PrCl + ArMgCl
From alkylzinc compounds (reductive
transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
)
A further method to synthesize Grignard reagents involves reaction of Mg with an organozinc compound. This method has been used to make adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the ...
-based Grignard reagents, which are, due to C-C coupling side reactions, difficult to make by the conventional method from the alkyl halide and Mg. The reductive transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
achieves:
:AdZnBr + Mg → AdMgBr + Zn
Testing Grignard reagents
Because Grignard reagents are so sensitive to moisture and oxygen, many methods have been developed to test the quality of a batch. Typical tests involve titrations with weighable, anhydrous protic reagents, e.g.menthol
Menthol is an organic compound, more specifically a monoterpenoid, made synthetically or obtained from the oils of corn mint, peppermint, or other mints. It is a waxy, clear or white crystalline substance, which is solid at room temperature ...
in the presence of a color-indicator. The interaction of the Grignard reagent with phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviate ...
or 2,2'-biquinoline causes a color change.
Reactions of Grignard reagents
With carbonyl compounds
Grignard reagents react with a variety ofcarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
derivatives.
 The most common application of Grignard reagents is the alkylation of aldehydes and ketones, i.e. ''the'' Grignard reaction:
The most common application of Grignard reagents is the alkylation of aldehydes and ketones, i.e. ''the'' Grignard reaction:
 Note that the
Note that the acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
function (a protected carbonyl) does not react.
Such reactions usually involve an aqueous acidic workup, though this step is rarely shown in reaction schemes. In cases where the Grignard reagent is adding to an aldehyde or a prochiral ketone, the Felkin-Anh model or Cram's Rule can usually predict which stereoisomer will be formed. With easily deprotonated 1,3-diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbony ...
s and related acidic substrates, the Grignard reagent RMgX functions merely as a base, giving the enolate anion and liberating the alkane RH.
Grignard reagents are nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
s in nucleophilic aliphatic substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). Th ...
s for instance with alkyl halides in a key step in industrial Naproxen production:


Reactions as a base
Grignard reagents serve as a base for protic substrates (this scheme does not show workup conditions, which typically includes water). Grignard reagents are basic and react with alcohols, phenols, etc. to givealkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s (ROMgBr). The phenoxide derivative is susceptible to formylation by paraformaldehyde
Paraformaldehyde (PFA) is the smallest polyoxymethylene, the polymerization product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition. Par ...
to give salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula (C7 H6 O2) C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily ...
.
Alkylation of metals and metalloids
Likeorganolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s, Grignard reagents are useful for forming carbon–heteroatom bonds.
:
Grignard reagents react with many metal-based electrophiles. For example, they undergo transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, bu ...
with cadmium chloride
Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (describ ...
(CdCl2) to give dialkylcadmium:
:2 RMgX + CdCl2 → R2Cd + 2 Mg(X)Cl
Schlenk equilibrium
Most Grignard reactions are conducted in ethereal solvents, especiallydiethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
and THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
. Grignard reagents react with 1,4-dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( ...
to give the diorganomagnesium compounds and insoluble coordination polymer MgX2(dioxane)2 and (R = organic group, X = halide):
:2 RMgX + dioxane R2Mg + MgX2(dioxane)2
This reaction exploits the Schlenk equilibrium, driving it toward the right.
Precursors to magnesiates
Grignard reagents react with organolithium compounds to give ate complexes (Bu = butyl): :BuMgBr + 3BuLi → LiMgBu3 + BuBrCoupling with organic halides
Grignard reagents do ''not'' typically react with organic halides, in contrast with their high reactivity with other main group halides. In the presence of metal catalysts, however, Grignard reagents participate in C-Ccoupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
s. For example, nonylmagnesium bromide reacts with methyl ''p''-chlorobenzoate to give ''p''-nonylbenzoic acid, in the presence of Tris(acetylacetonato)iron(III) (Fe(acac)3), after workup with NaOH to hydrolyze
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis i ...
the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
, shown as follows. Without the Fe(acac)3, the Grignard reagent would attack the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
group over the aryl halide.
nickel chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for che ...
in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) is also a good catalyst. Additionally, an effective catalyst for the couplings of alkyl halides is dilithium tetrachlorocuprate (Li2CuCl4), prepared by mixing lithium chloride (LiCl) and copper(II) chloride
Copper(II) chloride is the chemical compound with the chemical formula CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.
Both the anhydrous and the dihydrate forms occur naturally as the ve ...
(CuCl2) in THF. The Kumada-Corriu coupling gives access to ubstitutedstyrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
s.
Oxidation
Treatment of a Grignard reagent with oxygen gives the magnesium organoperoxide. Hydrolysis of this material yieldshydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
s or alcohol. These reactions involve radical
Radical may refer to:
Politics and ideology Politics
* Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe an ...
intermediates.
:
The simple oxidation of Grignard reagents to give alcohols is of little practical importance as yields are generally poor. In contrast, two-step sequence via a borane (''vide supra'') that is subsequently oxidized to the alcohol with hydrogen peroxide is of synthetic utility.
The synthetic utility of Grignard oxidations can be increased by a reaction of Grignard reagents with oxygen in presence of an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
to an ethylene extended alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
. This modification requires aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
or vinyl Grignards. Adding just the Grignard and the alkene does not result in a reaction demonstrating that the presence of oxygen is essential. The only drawback is the requirement of at least two equivalents of Grignard although this can partly be circumvented by the use of a dual Grignard system with a cheap reducing Grignard such as n-butylmagnesium bromide.

Elimination
In theBoord olefin synthesis The Boord olefin synthesis is an organic reaction forming alkenes from ethers carrying a halogen atom 2 carbons removed from the oxygen atom (β-halo-ethers) using a metal such as magnesium or zinc. The reaction, discovered by Cecil E. Boord i ...
, the addition of magnesium to certain β-haloethers results in an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
to the alkene. This reaction can limit the utility of Grignard reactions.

Industrial use
An example of the Grignard reaction is a key step in the (non-stereoselective) industrial production ofTamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and treat breast cancer in women and men. It is also being studied for other types of cancer. It has b ...
(currently used for the treatment of estrogen receptor positive breast cancer in women):
See also
* Dibutylmagnesium * Hauser baseGallery
References
Further reading
* *Mary McHale, "Grignard Reaction," Connexions, http://cnx.org/content/m15245/1.2/. 2007. *''Grignard knowledge: Alkyl coupling chemistry with inexpensive transition metals'' by Larry J. Westrum, Fine Chemistry November/December 2002, pp. 10–1Specialized literature
* * * * {{Authority control Organometallic chemistry Carbon-carbon bond forming reactions Carbon-heteroatom bond forming reactions Reagents for organic chemistry Magnesium Chemical tests Organomagnesium compounds