George S. Hammond on:
[Wikipedia]
[Google]
[Amazon]
George Simms Hammond (May 22, 1921 – October 5, 2005) was an American scientist and theoretical chemist who developed " Hammond's postulate", and fathered organic photochemistry,–the general theory of the geometric structure of the
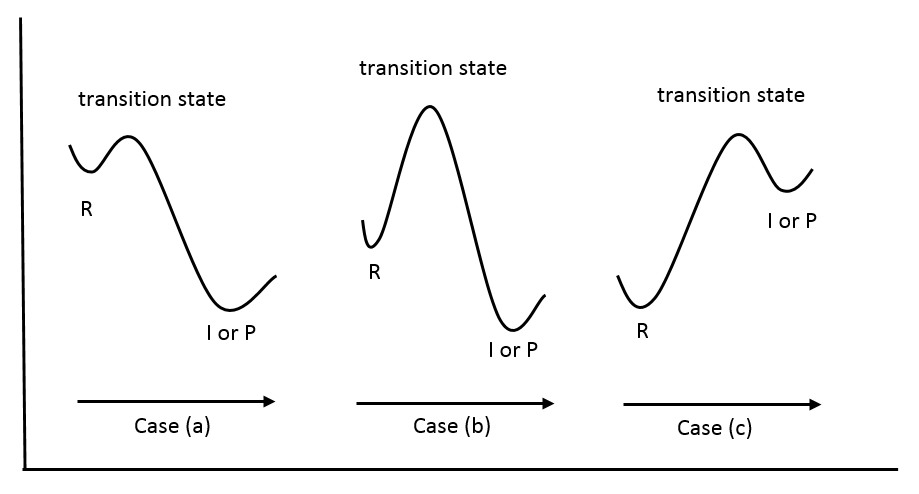 In case (a), which is an exothermic reaction, the energy of the transition state is closer in energy to that of the reactant than that of the intermediate or the product. Therefore, from the postulate, the structure of the transition state also more closely resembles that of the reactant. In case (b), the energy of the transition state is close to neither the reactant nor the product, making none of them a good structural model for the transition state. Further information would be needed in order to predict the structure or characteristics of the transition state. Case (c) depicts the potential diagram for an endothermic reaction, in which, according to the postulate, the transition state should more closely resemble that of the intermediate or the product.
Another significance of Hammond's postulate is that it permits us to discuss the structure of the transition state in terms of the reactants, intermediates, or products. In the case where the transition state closely resembles the reactants, the transition state is called “early” while a “late” transition state is the one that closely resembles the intermediate or the product.
An example of the “early” transition state is chlorination. Chlorination favors the products because it is an exothermic reaction, which means that the products are lower in energy than the reactants. When looking at the adjacent diagram (representation of an "early" transition state), one must focus on the transition state, which is not able to be observed during an experiment. To understand what is meant by an “early” transition state, the Hammond postulate represents a curve that shows the kinetics of this reaction. Since the reactants are higher in energy, the transition state appears to be right after the reaction starts.
An example of the “late” transition state is bromination. Bromination favors the reactants because it is an endothermic reaction, which means that the reactants are lower in energy than the products. Since the transition state is hard to observe, the postulate of bromination helps to picture the “late” transition state (see the representation of the "late" transition state). Since the products are higher in energy, the transition state appears to be right before the reaction is complete.
One other useful interpretation of the postulate often found in textbooks of
In case (a), which is an exothermic reaction, the energy of the transition state is closer in energy to that of the reactant than that of the intermediate or the product. Therefore, from the postulate, the structure of the transition state also more closely resembles that of the reactant. In case (b), the energy of the transition state is close to neither the reactant nor the product, making none of them a good structural model for the transition state. Further information would be needed in order to predict the structure or characteristics of the transition state. Case (c) depicts the potential diagram for an endothermic reaction, in which, according to the postulate, the transition state should more closely resemble that of the intermediate or the product.
Another significance of Hammond's postulate is that it permits us to discuss the structure of the transition state in terms of the reactants, intermediates, or products. In the case where the transition state closely resembles the reactants, the transition state is called “early” while a “late” transition state is the one that closely resembles the intermediate or the product.
An example of the “early” transition state is chlorination. Chlorination favors the products because it is an exothermic reaction, which means that the products are lower in energy than the reactants. When looking at the adjacent diagram (representation of an "early" transition state), one must focus on the transition state, which is not able to be observed during an experiment. To understand what is meant by an “early” transition state, the Hammond postulate represents a curve that shows the kinetics of this reaction. Since the reactants are higher in energy, the transition state appears to be right after the reaction starts.
An example of the “late” transition state is bromination. Bromination favors the reactants because it is an endothermic reaction, which means that the reactants are lower in energy than the products. Since the transition state is hard to observe, the postulate of bromination helps to picture the “late” transition state (see the representation of the "late" transition state). Since the products are higher in energy, the transition state appears to be right before the reaction is complete.
One other useful interpretation of the postulate often found in textbooks of
 Hammond's postulate can be used to examine the structure of the transition states of a
Hammond's postulate can be used to examine the structure of the transition states of a
 An E1 reaction consists of a unimolecular elimination, where the rate determining step of the mechanism depends on the removal of a single molecular species. This is a two-step mechanism. The more stable the carbocation intermediate is, the faster the reaction will proceed, favoring the products. Stabilization of the carbocation intermediate lowers the activation energy. The reactivity order is (CH3)3C- > (CH3)2CH- > CH3CH2- > CH3-.
An E1 reaction consists of a unimolecular elimination, where the rate determining step of the mechanism depends on the removal of a single molecular species. This is a two-step mechanism. The more stable the carbocation intermediate is, the faster the reaction will proceed, favoring the products. Stabilization of the carbocation intermediate lowers the activation energy. The reactivity order is (CH3)3C- > (CH3)2CH- > CH3CH2- > CH3-.
 Furthermore, studies describe a typical kinetic resolution process that starts out with two enantiomers that are energetically equivalent and, in the end, forms two energy-inequivalent intermediates, referred to as diastereomers. According to Hammond's postulate, the more stable diastereomer is formed faster.
Furthermore, studies describe a typical kinetic resolution process that starts out with two enantiomers that are energetically equivalent and, in the end, forms two energy-inequivalent intermediates, referred to as diastereomers. According to Hammond's postulate, the more stable diastereomer is formed faster.
 The relationship between Hammond's postulate and the BEP principle can be understood by considering a SN1 reaction. Although two transition states occur during a SN1 reaction (dissociation of the leaving group and then attack by the nucleophile), the dissociation of the leaving group is almost always the
The relationship between Hammond's postulate and the BEP principle can be understood by considering a SN1 reaction. Although two transition states occur during a SN1 reaction (dissociation of the leaving group and then attack by the nucleophile), the dissociation of the leaving group is almost always the
Photographs of George S. Hammond from the UC Santa Cruz Library's Digital Collections
{{DEFAULTSORT:Hammond, George S. 1921 births 2005 deaths National Medal of Science laureates 20th-century American chemists Bates College alumni Harvard University alumni Chemical kinetics Physical organic chemistry University of California, Los Angeles alumni Iowa State University faculty California Institute of Technology faculty University of California, Santa Cruz faculty American expatriates in the United Kingdom American expatriates in Switzerland
transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
in an organic chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
. Hammond's research is also known for its influence on the philosophy of science
Philosophy of science is a branch of philosophy concerned with the foundations, methods, and implications of science. The central questions of this study concern what qualifies as science, the reliability of scientific theories, and the ult ...
. His research garnered him the Norris Award in 1968, the Priestley Medal in 1976, the National Medal of Science in 1994, and the Othmer Gold Medal
The Othmer Gold Medal recognizes outstanding individuals who contributed to progress in chemistry and science through their activities in areas including innovation, entrepreneurship, research, education, public understanding, legislation, and ph ...
in 2003. He served as the executive chairman of the Allied Chemical Corporation
Allied Corp. was a major American company with operations in the chemical, aerospace, automotive, oil and gas industries. It was initially formed in 1920 as the Allied Chemical and Dye Corporation as an amalgamation of five chemical companies. In ...
from 1979 to 1989.
He was a chemist at the California Institute of Technology
The California Institute of Technology (branded as Caltech or CIT)The university itself only spells its short form as "Caltech"; the institution considers other spellings such a"Cal Tech" and "CalTech" incorrect. The institute is also occasional ...
, and subsequently headed both the Departments of Chemistry and Chemical Engineering at the university. He conducted research at the University of Oxford
, mottoeng = The Lord is my light
, established =
, endowment = £6.1 billion (including colleges) (2019)
, budget = £2.145 billion (2019–20)
, chancellor ...
and University of Basel
The University of Basel (Latin: ''Universitas Basiliensis'', German: ''Universität Basel'') is a university in Basel, Switzerland. Founded on 4 April 1460, it is Switzerland's oldest university and among the world's oldest surviving universit ...
as a Guggenheim Fellow
Guggenheim Fellowships are grants that have been awarded annually since by the John Simon Guggenheim Memorial Foundation to those "who have demonstrated exceptional capacity for productive scholarship or exceptional creative ability in the a ...
and National Science Foundation Fellow, respectively. He served as the foreign secretary of the National Academy of Sciences from 1974 to 1978.
A native of Maine
Maine () is a state in the New England and Northeastern regions of the United States. It borders New Hampshire to the west, the Gulf of Maine to the southeast, and the Canadian provinces of New Brunswick and Quebec to the northeast and ...
, he was born and raised in Auburn; he attended nearby Bates College
Bates College () is a private liberal arts college in Lewiston, Maine. Anchored by the Historic Quad, the campus of Bates totals with a small urban campus which includes 33 Victorian Houses as some of the dormitories. It maintains of nature p ...
in Lewiston, Maine
Lewiston (; ; officially the City of Lewiston, Maine) is the second largest city in Maine and the most central city in Androscoggin County. The city lies halfway between Augusta, the state's capital, and Portland, the state's most populous ci ...
, where he graduated magna cum laude with a B.S.
A Bachelor of Science (BS, BSc, SB, or ScB; from the Latin ') is a bachelor's degree awarded for programs that generally last three to five years.
The first university to admit a student to the degree of Bachelor of Science was the University ...
in chemistry in 1943. He completed his doctorate at Harvard University
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of high ...
in 1947, under the mentorship of Paul Doughty Bartlett, and a postdoctorate at University of California, Los Angeles
The University of California, Los Angeles (UCLA) is a public land-grant research university in Los Angeles, California. UCLA's academic roots were established in 1881 as a teachers college then known as the southern branch of the California S ...
with Saul Winstein in 1948.
Early life and education
George Simmons Hammond was born on May 22, 1921 inAuburn, Maine
Auburn is a city in south-central Maine within the United States. The city serves as the county seat of Androscoggin County. The population was 24,061 at the 2020 census. Auburn and its sister city Lewiston are known locally as the Twin Cities ...
. Growing up in Auburn his family were charged with the operation of the neighborhood dairy farm on Hardscrapple Road. His father died when Hammond was thirteen. He was the oldest of seven children and was raised by a single mother. From an early age Hammond was charged with running the day-to-day operations of the dairy farm with his mother and older siblings. Hammond's parents were college graduates, but disliked the local schools in Auburn. As a result, he was homeschooled until the sixth grade. Afterwards, he was educated at various Auburn public schools before graduating in 1938. After graduating he took a gap year
A gap year, also known as a sabbatical year, is typically a year-long break before or after college/university during which students engage in various educational and developmental activities, such as travel or some type of regular work. Gap yea ...
to continue operating his dairy farm. After his educational hiatus he applied to and was accepted into Bates College
Bates College () is a private liberal arts college in Lewiston, Maine. Anchored by the Historic Quad, the campus of Bates totals with a small urban campus which includes 33 Victorian Houses as some of the dormitories. It maintains of nature p ...
, in Lewiston, Maine
Lewiston (; ; officially the City of Lewiston, Maine) is the second largest city in Maine and the most central city in Androscoggin County. The city lies halfway between Augusta, the state's capital, and Portland, the state's most populous ci ...
. He graduated with a Bachelors of Science in chemistry magna cum laude and Phi Beta Kappa
The Phi Beta Kappa Society () is the oldest academic honor society in the United States, and the most prestigious, due in part to its long history and academic selectivity. Phi Beta Kappa aims to promote and advocate excellence in the liberal ...
in January 1943.
Early career
Upon graduating from college, Hammond took a position as achemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe t ...
at Rohm and Haas in Philadelphia, Pennsylvania
Philadelphia, often called Philly, is the largest city in the Commonwealth of Pennsylvania, the sixth-largest city in the U.S., the second-largest city in both the Northeast megalopolis and Mid-Atlantic regions after New York City. Sinc ...
. After some months on the job he quit to pursue graduate studies at Harvard University
Harvard University is a private Ivy League research university in Cambridge, Massachusetts. Founded in 1636 as Harvard College and named for its first benefactor, the Puritan clergyman John Harvard, it is the oldest institution of high ...
, where he received a Masters of Science (M.S.) and Doctor of Philosophy
A Doctor of Philosophy (PhD, Ph.D., or DPhil; Latin: or ') is the most common degree at the highest academic level awarded following a course of study. PhDs are awarded for programs across the whole breadth of academic fields. Because it is ...
(Ph.D.). His thesis, ''Inhibition of the Polymerization of Allylacetate'', was supervised by Paul Doughty Bartlett. Hammond then moved to Los Angeles, California
Los Angeles ( ; es, Los Ángeles, link=no , ), often referred to by its initials L.A., is the largest city in the state of California and the second most populous city in the United States after New York City, as well as one of the world' ...
to study intermolecular compounds at UCLA
The University of California, Los Angeles (UCLA) is a public land-grant research university in Los Angeles, California. UCLA's academic roots were established in 1881 as a teachers college then known as the southern branch of the California ...
.
Career in academia
His academic career began in 1948 with a teaching position atIowa State College
Iowa State University of Science and Technology (Iowa State University, Iowa State, or ISU) is a public land-grant research university in Ames, Iowa. Founded in 1858 as the Iowa Agricultural College and Model Farm, Iowa State became one of the n ...
; he served as Assistant Professor
Assistant Professor is an academic rank just below the rank of an associate professor used in universities or colleges, mainly in the United States and Canada.
Overview
This position is generally taken after earning a doctoral degree
A docto ...
of Chemistry. In his capacity there he published his eponymous postulate which is now widely known as the most important publication in the field of organic photochemistry. He moved to the University of Oxford
, mottoeng = The Lord is my light
, established =
, endowment = £6.1 billion (including colleges) (2019)
, budget = £2.145 billion (2019–20)
, chancellor ...
and University of Basel
The University of Basel (Latin: ''Universitas Basiliensis'', German: ''Universität Basel'') is a university in Basel, Switzerland. Founded on 4 April 1460, it is Switzerland's oldest university and among the world's oldest surviving universit ...
as a Guggenheim Fellow
Guggenheim Fellowships are grants that have been awarded annually since by the John Simon Guggenheim Memorial Foundation to those "who have demonstrated exceptional capacity for productive scholarship or exceptional creative ability in the a ...
and National Science Foundation Fellow, respectively. In 1958, he moved to the California Institute of Technology
The California Institute of Technology (branded as Caltech or CIT)The university itself only spells its short form as "Caltech"; the institution considers other spellings such a"Cal Tech" and "CalTech" incorrect. The institute is also occasional ...
as a Professor of Organic Chemistry. Later he was appointed the Arthur Amos Noyes Professor of Chemistry and subsequently went on to lead the Departments of Chemistry and Chemical Engineering. After 14 years teaching and serving as an academic administrator at Caltech he moved in 1972 to the University of California Santa Cruz
The University of California, Santa Cruz (UC Santa Cruz or UCSC) is a public university, public Land-grant university, land-grant research university in Santa Cruz, California. It is one of the ten campuses in the University of California syste ...
. At University of California Santa Cruz he served as both a professor and the Chancellor of the natural sciences.
Life outside of academia
Aside from the academic world, during all these years, George Hammond, "made many public speeches on controversial themes, both political (e.g., the invasion of Cambodia, delivered in 1971 at a public rally on Caltech's Olive Walk) and scientific (e.g., the future of chemistry)" Many of these controversial speeches affected his career negatively. For example, after his speech at Olive Walk, president Richard Nixon's administration removed his name from nomination for a major NSF post. Nevertheless, he did not back down and continued to criticize the government, and not limiting to delivering speeches, he wrote a letter to the editor of a newspaper saying: “A June 30 front-page article describes the potential bonanza in arms sales to new members as the North Atlantic Treaty Organization expands. I was favorably inclined toward expansion because of my naive assumption that bringing most of the nations of Europe and North America together as a cooperating group would decrease the likelihood of war. I cannot believe this will be the case if a prerequisite for entry is that countries buy new armaments from present members. At whom will the guns be aimed? Russia? Then we will probably re-create the cold war." The way this excerpt was written says many things about George Hammond, starting with his passionate character. Hammond fought for everything he believed in. He cared about his nation and he was also a little reckless about the consequences he could suffer for defying the government. Also, in the excerpt, a sarcastic side of Hammond can be perceived, a man of strong character with the ability to recognize when he is wrong.Later pursuits
He was appointed as the Foreign Secretary of the National Academy of Sciences in 1974 and served for one term retiring in 1978. He also gave notable speeches on political issues such as the invasion of Cambodia, and various topics on Chemistry. The talks he gave sometimes had negative impacts on his life, exemplified by Nixon's withdrawal of his name for majorNational Science Foundation
The National Science Foundation (NSF) is an independent agency of the United States government that supports fundamental research and education in all the non-medical fields of science and engineering. Its medical counterpart is the National ...
positions. In 1979 he retired from academia and joined the Allied Chemical Corporation
Allied Corp. was a major American company with operations in the chemical, aerospace, automotive, oil and gas industries. It was initially formed in 1920 as the Allied Chemical and Dye Corporation as an amalgamation of five chemical companies. In ...
as Executive Chairman, serving for ten years. He retired from this capacity and all others after his tenure concluded.
Scientific career
Hammond's postulate
George Hammond published ahypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous obse ...
in physical organic chemistry
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical c ...
which describes the geometric structure of the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
in an organic chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
in his publication, Hammond's principle.
His 1955 publication asserted:
:"If two states, as, for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures."Therefore, the geometric structure of a state can be predicted by comparing its energy to the species neighboring it along the
reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecu ...
. For example, in an ''exothermic'' reaction the transition state is closer in energy to the reactants than to the products. Therefore, the transition state will be more geometrically similar to the reactants than to the products. In contrast, however, in an ''endothermic'' reaction the transition state is closer in energy to the ''products'' than to the reactants. So, according to Hammond's postulate the structure of the transition state would resemble the products more than the reactants. This type of comparison is especially useful because most transition states cannot be characterized experimentally.
Hammond's postulate also helps to explain and rationalize the Bell–Evans–Polanyi principle In physical chemistry, the Evans–Polanyi principle (also referred to as the Bell–Evans–Polanyi principle, Brønsted–Evans–Polanyi principle, or Evans–Polanyi–Semenov principle) observes that the difference in activation energy between ...
. Namely, this principle describes the experimental observation that the rate of a reaction, and therefore its activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
, is affected by the enthalpy change of that reaction. Hammond's postulate explains this observation by describing how varying the enthalpy of a reaction would also change the structure of the transition state. In turn, this change in geometric structure would alter the energy of the transition state, and therefore the activation energy and reaction rate as well.
The postulate has also been used to predict the shape of reaction coordinate diagrams. For example, electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
s involves a distinct intermediate and two less well defined states. By measuring the effects of aromatic substituents and applying Hammond's postulate it was concluded that the rate-determining step involves formation of a transition state that should resemble the intermediate complex.
During the 1940s and 1950s, chemists had trouble explaining why even slight changes in the reactants caused significant differences in the rate and product distributions of a reaction. In 1955 George S. Hammond, a young professor at Iowa State University
Iowa State University of Science and Technology (Iowa State University, Iowa State, or ISU) is a public land-grant research university in Ames, Iowa. Founded in 1858 as the Iowa Agricultural College and Model Farm, Iowa State became one of the ...
, postulated that transition-state theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.
...
could be used to qualitatively explain the observed structure-reactivity relationships. Notably, John E. Leffler of Florida State University proposed a similar idea in 1953. However, Hammond's version has received more attention since its qualitative nature was easier to understand and employ than Leffler's complex mathematical equations. Hammond's postulate is sometimes called the Hammond-Leffler postulate to give credit to both scientists.
Interpreting the postulate
Effectively, the postulate states that the structure of a transition state resembles that of the species nearest to it in free energy. This can be explained with reference to potential energy diagrams:organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
is the following:
:''Assume that the transition states for reactions involving unstable intermediates can be closely approximated by the intermediates themselves.''
This interpretation ignores extremely exothermic and endothermic reactions which are relatively unusual and relates the transition state to the intermediates which are usually the most unstable.
Structure of transition states
SN1 reactions
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. ...
. In particular, the dissociation of the leaving group is the first transition state in a SN1 reaction. The stabilities of the carbocations formed by this dissociation are known to follow the trend tertiary > secondary > primary > methyl.
Therefore, since the tertiary carbocation is relatively stable and therefore close in energy to the R-X reactant, then the tertiary transition state will have a structure that is fairly similar to the R-X reactant. In terms of the graph of reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecu ...
versus energy, this is shown by the fact that the tertiary transition state is further to the left than the other transition states. In contrast, the energy of a methyl carbocation is very high, and therefore the structure of the transition state is more similar to the intermediate carbocation than to the R-X reactant. Accordingly, the methyl transition state is very far to the right.
SN2 reactions
Substitution, nucleophilic bimolecular reactions are concerted reactions where both the nucleophile and substrate are involved in the rate limiting step. Since this reaction is concerted, the reaction occurs in one step, where the bonds are broken, while new bonds are formed. Therefore, to interpret this reaction, it is important to look at the transition state, which resembles the concerted rate limiting step. In the "Depiction of SN2 Reaction" figure, the nucleophile forms a new bond to the carbon, while the halide (L) bond is broken.E1 reactions
 An E1 reaction consists of a unimolecular elimination, where the rate determining step of the mechanism depends on the removal of a single molecular species. This is a two-step mechanism. The more stable the carbocation intermediate is, the faster the reaction will proceed, favoring the products. Stabilization of the carbocation intermediate lowers the activation energy. The reactivity order is (CH3)3C- > (CH3)2CH- > CH3CH2- > CH3-.
An E1 reaction consists of a unimolecular elimination, where the rate determining step of the mechanism depends on the removal of a single molecular species. This is a two-step mechanism. The more stable the carbocation intermediate is, the faster the reaction will proceed, favoring the products. Stabilization of the carbocation intermediate lowers the activation energy. The reactivity order is (CH3)3C- > (CH3)2CH- > CH3CH2- > CH3-.
 Furthermore, studies describe a typical kinetic resolution process that starts out with two enantiomers that are energetically equivalent and, in the end, forms two energy-inequivalent intermediates, referred to as diastereomers. According to Hammond's postulate, the more stable diastereomer is formed faster.
Furthermore, studies describe a typical kinetic resolution process that starts out with two enantiomers that are energetically equivalent and, in the end, forms two energy-inequivalent intermediates, referred to as diastereomers. According to Hammond's postulate, the more stable diastereomer is formed faster.
E2 reactions
Elimination, bimolecular reactions are one step, concerted reaction where both base and substrate participate in the rate limiting step. In an E2 mechanism, a base takes a proton near the leaving group, forcing the electrons down to make a double bond, and forcing off the leaving group-all in one concerted step. The rate law depends on the first order concentration of two reactants, making it a 2nd order (bimolecular) elimination reaction. Factors that affect the rate determining step are stereochemistry, leaving groups, and base strength. A theory, for an E2 reaction, by Joseph Bunnett suggests the lowest pass through the energy barrier between reactants and products is gained by an adjustment between the degrees of Cβ-H and Cα-X rupture at the transition state. The adjustment involves much breaking of the bond more easily broken, and a small amount of breaking of the bond which requires more energy. This conclusion by Bunnett is a contradiction from the Hammond postulate. The Hammond postulate is the opposite of what Bunnett theorized. In the transition state of a bond breaking step it involves little breaking when the bond is easily broken and much breaking when it is difficult to break. Despite these differences, the two postulates are not in conflict since they are concerned with different sorts of processes. Hammond focuses on reaction steps where one bond is made or broken, or the breaking of two or more bonds occur simultaneously. The E2 theory transition state concerns a process when bond formation or breaking are not simultaneous.Kinetics and the Bell-Evans-Polanyi principle
Technically, Hammond's postulate only describes the geometric structure of a chemical reaction. However, Hammond's postulate indirectly gives information about the rate, kinetics, andactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
of reactions. Hence, it gives a theoretical basis for the understanding the Bell-Evans-Polanyi principle, which describes the experimental observation that the enthalpy change and rate of similar reactions were usually correlated.
rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
. Hence, the activation energy and therefore rate of the reaction will depend only upon the dissociation step.
First, consider the reaction at secondary and tertiary carbons. As the BEP principle notes, experimentally SN1 reactions at tertiary carbons are faster than at secondary carbons. Therefore, by definition, the transition state for tertiary reactions will be at a lower energy than for secondary reactions. However, the BEP principle cannot justify why the energy is lower.
Using Hammond's postulate, the lower energy of the tertiary transition state means that its structure is relatively closer to its reactants R(tertiary)-X than to the carbocation "product" when compared to the secondary case. Thus, the tertiary transition state will be more geometrically similar to the R(tertiary)-X reactants than the secondary transition state is to its R(secondary)-X reactants. Hence, if the tertiary transition state is close in structure to the (low energy) reactants, then it will also be lower in energy because structure determines energy. Likewise, if the secondary transition state is more similar to the (high energy) carbocation "product," then it will be higher in energy.Applying the postulate
Hammond's postulate is useful for understanding the relationship between the rate of a reaction and the stability of the products. While the rate of a reaction depends just on theactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
(often represented in organic chemistry as ΔG‡ “delta G double dagger”), the final ratios of products in chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
depends only on the standard free-energy change Δ''G'' (“delta ''G''”). The ratio of the final products at equilibrium corresponds directly with the stability of those products.
Hammond's postulate connects the rate of a reaction process with the structural features of those states that form part of it, by saying that the molecular reorganizations have to be small in those steps that involve two states that are very close in energy. This gave birth to the structural comparison between the starting materials, products, and the possible "stable intermediates" that led to the understanding that the most stable product is not always the one that is favored in a reaction process.
Critical acclaim and question
Hammond's postulate is especially important when looking at therate-limiting step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of th ...
of a reaction. However, one must be cautious when examining a multistep reaction or one with the possibility of rearrangements during an intermediate stage. In some cases, the final products appear in skewed ratios in favor of a more unstable product (called the kinetic product) rather than the more stable product (the thermodynamic product). In this case one must examine the rate-limiting step and the intermediates. Often, the rate-limiting step is the initial formation of an unstable species such as a carbocation. Then, once the carbocation is formed, subsequent rearrangements can occur. In these kinds of reactions, especially when run at lower temperatures, the reactants simply react before the rearrangements necessary to form a more stable intermediate have time to occur. At higher temperatures when microscopic reversal is easier, the more stable thermodynamic product is favored because these intermediates have time to rearrange. Whether run at high or low temperatures, the mixture of the kinetic and thermodynamic products eventually reach the same ratio, one in favor of the more stable thermodynamic product, when given time to equilibrate due to microreversal.
Personal
Hammond married Marian Reese in 1945, and had five children with her. The couple divorced in 1975, and he was remarried soon after to Eve Menger. He had two children with Eve.Awards and honors
* Norris Award (1968) * Priestley Medal (1976) * Golden Plate Award of theAmerican Academy of Achievement
The American Academy of Achievement, colloquially known as the Academy of Achievement, is a non-profit educational organization that recognizes some of the highest achieving individuals in diverse fields and gives them the opportunity to meet ...
(1976)
* National Medal of Science
The National Medal of Science is an honor bestowed by the President of the United States to individuals in science and engineering who have made important contributions to the advancement of knowledge in the fields of behavioral and social scienc ...
(1994)
* Glenn T. Seaborg Medal (1994)
* Othmer Gold Medal
The Othmer Gold Medal recognizes outstanding individuals who contributed to progress in chemistry and science through their activities in areas including innovation, entrepreneurship, research, education, public understanding, legislation, and ph ...
(2003)
See also
* Bema Hapothle * Curtin-Hammett principle *Microscopic reversibility The principle of microscopic reversibility in physics and chemistry is twofold:
* First, it states that the microscopic detailed dynamics of particles and fields is time-reversible because the microscopic equations of motion are symmetric with resp ...
* Bell-Evans-Polanyi principle
References
Further reading
*External links
Photographs of George S. Hammond from the UC Santa Cruz Library's Digital Collections
{{DEFAULTSORT:Hammond, George S. 1921 births 2005 deaths National Medal of Science laureates 20th-century American chemists Bates College alumni Harvard University alumni Chemical kinetics Physical organic chemistry University of California, Los Angeles alumni Iowa State University faculty California Institute of Technology faculty University of California, Santa Cruz faculty American expatriates in the United Kingdom American expatriates in Switzerland