Genital warts on:
[Wikipedia]
[Google]
[Amazon]
Genital warts are a
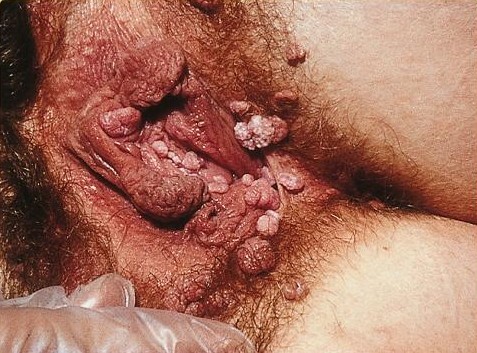

 They may be found anywhere in the anal or genital area, and are frequently found on external surfaces of the body, including the penile shaft,
They may be found anywhere in the anal or genital area, and are frequently found on external surfaces of the body, including the penile shaft,
REPORT TO CONGRESS: Prevention of Genital Human Papillomavirus Infection
). Warts can also spontaneously regress (with or without treatment). Traditional theories postulated that the virus remained in the body for a lifetime. However, studies using sensitive DNA techniques have shown that through immunological response, the virus can either be cleared or suppressed to levels below what
sexually transmitted infection
Sexually transmitted infections (STIs), also referred to as sexually transmitted diseases (STDs) and the older term venereal diseases, are infections that are spread by sexual activity, especially vaginal intercourse, anal sex, and ora ...
caused by certain types of human papillomavirus
Human papillomavirus infection (HPV infection) is caused by a DNA virus from the '' Papillomaviridae'' family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. In some cases, an HPV infection persists and r ...
(HPV). They are generally pink in color and project out from the surface of the skin. Usually they cause few symptoms, but can occasionally be painful. Typically they appear one to eight months following exposure. Wart
Warts are typically small, rough, hard growths that are similar in color to the rest of the skin. They typically do not result in other symptoms, except when on the bottom of the feet, where they may be painful. While they usually occur on the ...
s are the most easily recognized symptom of genital HPV infection.
HPV types 6 and 11 are responsible for causing majority of genital warts whereas HPV types 16, 18, 31, 33, and 35 are also occasionally found. It is spread through direct skin-to-skin contact, usually during oral
The word oral may refer to:
Relating to the mouth
* Relating to the mouth, the first portion of the alimentary canal that primarily receives food and liquid
** Oral administration of medicines
** Oral examination (also known as an oral exam or or ...
, genital
A sex organ (or reproductive organ) is any part of an animal or plant that is involved in sexual reproduction. The reproductive organs together constitute the reproductive system. In animals, the testis in the male, and the ovary in the female, a ...
, or anal sex
Anal sex or anal intercourse is generally the insertion and thrusting of the erect penis into a person's anus, or anus and rectum, for sexual pleasure.Sepages 270–271for anal sex information, anpage 118for information about the clitoris. ...
with an infected partner. Diagnosis is generally based on symptoms and can be confirmed by biopsy
A biopsy is a medical test commonly performed by a surgeon, interventional radiologist, or an interventional cardiologist. The process involves extraction of sample cells or tissues for examination to determine the presence or extent of a dise ...
. The types of HPV that cause cancer are not the same as those that cause warts.
Some HPV vaccine
Human papillomavirus (HPV) vaccines are vaccines that prevent infection by certain types of human papillomavirus (HPV). Available HPV vaccines protect against either two, four, or nine types of HPV. All HPV vaccines protect against at least HP ...
s can prevent genital warts as may condoms
A condom is a sheath-shaped barrier device used during sexual intercourse to reduce the probability of pregnancy or a sexually transmitted infection (STI). There are both male and female condoms. With proper use—and use at every act of inte ...
. Treatment options include creams such as podophyllin, imiquimod, and trichloroacetic acid. Cryotherapy
Cryotherapy, sometimes known as cold therapy, is the local or general use of low temperatures in medical therapy. Cryotherapy may be used to treat a variety of tissue lesions. The most prominent use of the term refers to the surgical treatment, s ...
or surgery may also be an option. After treatment warts often resolve within six months. Without treatment, in up to a third of cases they resolve on their own.
About 1% of people in the United States have genital warts. Many people, however, are infected and do not have symptoms. Without vaccination nearly all sexually active people will get some type of HPV at one point in their lives. The disease has been known at least since the time of Hippocrates
Hippocrates of Kos (; grc-gre, Ἱπποκράτης ὁ Κῷος, Hippokrátēs ho Kôios; ), also known as Hippocrates II, was a Greek physician of the classical period who is considered one of the most outstanding figures in the history o ...
in 300 BC.
Signs and symptoms


 They may be found anywhere in the anal or genital area, and are frequently found on external surfaces of the body, including the penile shaft,
They may be found anywhere in the anal or genital area, and are frequently found on external surfaces of the body, including the penile shaft, scrotum
The scrotum or scrotal sac is an anatomical male reproductive structure located at the base of the penis that consists of a suspended dual-chambered sac of skin and smooth muscle. It is present in most terrestrial male mammals. The scrotum co ...
, or labia majora
The labia majora (singular: ''labium majus'') are two prominent longitudinal cutaneous folds that extend downward and backward from the mons pubis to the perineum. Together with the labia minora they form the labia of the vulva.
The labia maj ...
of the vagina
In mammals, the vagina is the elastic, muscular part of the female genital tract. In humans, it extends from the vestibule to the cervix. The outer vaginal opening is normally partly covered by a thin layer of mucosal tissue called the hymen ...
. They can also occur on internal surfaces like the opening to the urethra, inside the vagina, on the cervix
The cervix or cervix uteri (Latin, 'neck of the uterus') is the lower part of the uterus (womb) in the human female reproductive system. The cervix is usually 2 to 3 cm long (~1 inch) and roughly cylindrical in shape, which changes during ...
, or in the anus.
They can be as small as 1-5mm in diameter, but can also grow or spread into large masses in the genital or anal area. In some cases they look like small stalks. They may be hard ("keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up Scale (anatomy), scales, hair, Nail ...
ized") or soft. Their color can be variable, and sometimes they may bleed.
In most cases, there are no symptoms of HPV infection other than the warts themselves. Sometimes warts may cause itching, redness, or discomfort, especially when they occur around the anus. Although they are usually without other physical symptoms, an outbreak of genital warts may cause psychological distress, such as anxiety
Anxiety is an emotion which is characterized by an unpleasant state of inner turmoil and includes feelings of dread over anticipated events. Anxiety is different than fear in that the former is defined as the anticipation of a future threat wh ...
, in some people.
Causes
Transmission
HPV is most commonly transmitted through penetrative sex. While HPV can also be transmitted via non-penetrative sexual activity, it is less transmissible than via penetrative sex. There is conflicting evidence about the effect ofcondoms
A condom is a sheath-shaped barrier device used during sexual intercourse to reduce the probability of pregnancy or a sexually transmitted infection (STI). There are both male and female condoms. With proper use—and use at every act of inte ...
on transmission of low-risk HPV. Some studies have suggested that they are effective at reducing transmission. Other studies suggest that condoms are not effective at preventing transmission of the low-risk HPV variants that cause genital warts. The effect of condoms on HPV transmission may also be sex-dependent; there is some evidence that condoms are more effective at preventing infection of males than of females.
The types of HPV that cause warts are highly transmissible. Roughly three out of four unaffected partners of patients with warts develop them within eight months. Other studies of partner concordance suggest that the presence of visible warts may be an indicator of increased infectivity; HPV concordance rates are higher in couples where one partner has visible warts.
Latency and recurrence
Although 90% of HPV infections are cleared by the body within two years of infection, it is possible for infected cells to undergo a latency (quiet) period, with the first occurrence or a recurrence of symptoms happening months or years later. Latent HPV, even with no outward symptoms, is still transmissible to a sexual partner. If an individual has unprotected sex with an infected partner, there is a 70% chance that he or she will also become infected. In individuals with a history of previous HPV infection, the appearance of new warts may be either from a new exposure to HPV, or from a recurrence of the previous infection. As many as one-third of people with warts will experience a recurrence.Children
Anal or genital warts may be transmitted during birth. The presence of wart-like lesions on the genitals of young children has been suggested as an indicator of sexual abuse. However, genital warts can sometimes result fromautoinoculation Autoinoculation is derived from the Latin root words "autos" and "inoculate" that mean "self implanting" or "self infection" or "implanting something from oneself". Autoinoculation can refer to both beneficial medical procedures (e.g. vaccination) a ...
by warts elsewhere on the body, such as from the hands. It has also been reported from sharing of swimsuits, underwear, or bath towels, and from non-sexual touching during routine care such as diapering. Genital warts in children are less likely to be caused by HPV subtypes 6 and 11 than adults, and more likely to be caused by HPV types that cause warts elsewhere on the body ("cutaneous types"). Surveys of pediatricians who are child abuse specialists suggest that in children younger than 4 years old, there is no consensus on whether the appearance of new anal or genital warts, by itself, can be considered an indicator of sexual abuse.
Diagnosis
The diagnosis of genital warts is most often made visually, but may require confirmation bybiopsy
A biopsy is a medical test commonly performed by a surgeon, interventional radiologist, or an interventional cardiologist. The process involves extraction of sample cells or tissues for examination to determine the presence or extent of a dise ...
in some cases. Smaller warts may occasionally be confused with molluscum contagiosum
Molluscum contagiosum (MC), sometimes called water warts, is a viral infection of the skin that results in small raised pink lesions with a dimple in the center. They may become itchy or sore, and occur singularly or in groups. Any area of the sk ...
.
Genital warts, histopathologically
Histopathology (compound of three Greek words: ''histos'' "tissue", πάθος ''pathos'' "suffering", and -λογία ''-logia'' "study of") refers to the microscopic examination of tissue in order to study the manifestations of disease. Spec ...
, characteristically rise above the skin surface due to enlargement of the dermal papillae
The dermis or corium is a layer of skin between the epidermis (with which it makes up the cutis) and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided in ...
, have parakeratosis and the characteristic nuclear changes typical of HPV
Human papillomavirus infection (HPV infection) is caused by a DNA virus from the ''Papillomaviridae'' family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. In some cases, an HPV infection persists and res ...
infections (nuclear enlargement with perinuclear clearing).
DNA tests are available for diagnosis of high-risk HPV infections. Because genital warts are caused by low-risk HPV types, DNA tests cannot be used for diagnosis of genital warts or other low-risk HPV infections.
Some practitioners use an acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
solution to identify smaller warts ("subclinical lesions"), but this practice is controversial. Because a diagnosis made with acetic acid will not meaningfully affect the course of the disease, and cannot be verified by a more specific test, a 2007 UK guideline advises against its use.
Prevention
Gardasil (sold by Merck & Co.) is avaccine
A vaccine is a biological preparation that provides active acquired immunity to a particular infectious or malignant disease. The safety and effectiveness of vaccines has been widely studied and verified.
that protects against human papillomavirus
Human papillomavirus infection (HPV infection) is caused by a DNA virus from the '' Papillomaviridae'' family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. In some cases, an HPV infection persists and r ...
types 6, 11, 16 and 18. Types 6 and 11 cause genital warts, while 16 and 18 cause cervical cancer
Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal ...
. The vaccine is preventive, not therapeutic, and must be given before exposure to the virus type to be effective, ideally before the beginning of sexual activity. The vaccine is approved by the US Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
for use in both males and females as early as 9 years of age.
In the UK, Gardasil replaced Cervarix in September 2012 for reasons unrelated to safety. Cervarix had been used routinely in young females from its introduction in 2008, but was only effective against the high-risk HPV types 16 and 18, neither of which typically causes warts.
Management
There is no cure for HPV. Existing treatments are directed towards the removal of visible warts, but these may also regress on their own without any therapy. There is no evidence to suggest that removing visible warts reduces transmission of the underlying HPV infection. As many as 80% of people with HPV will clear the infection within 18 months. A healthcare practitioner may offer one of several ways to treat warts, depending on their number, sizes, locations, or other factors. All treatments can cause depigmentation, itching, pain, orscar
A scar (or scar tissue) is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs, and tissues of the body. Thus, scarring is a ...
ring.
Treatments can be classified as either physically ablative
In grammar, the ablative case (pronounced ; sometimes abbreviated ) is a grammatical case for nouns, pronouns, and adjectives in the grammars of various languages; it is sometimes used to express motion away from something, among other uses. ...
, or topical
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes ...
agents. Physically ablative therapies are considered more effective at initial wart removal, but like all therapies have significant recurrence rates.
Many therapies, including folk remedies
Traditional medicine (also known as indigenous medicine or folk medicine) comprises medical aspects of traditional knowledge that developed over generations within the folk beliefs of various societies, including indigenous peoples, before the ...
, have been suggested for treating genital warts, some of which have little evidence
Evidence for a proposition is what supports this proposition. It is usually understood as an indication that the supported proposition is true. What role evidence plays and how it is conceived varies from field to field.
In epistemology, evidenc ...
to suggest they are effective or safe. Those listed here are ones mentioned in national or international practice guidelines as having some basis in evidence for their use.
Physical ablation
Physically ablative methods are more likely to be effective on keratinized warts. They are also most appropriate for patients with fewer numbers of relatively smaller warts. * Simple excision, such as with scissors underlocal anesthesia
Local anesthesia is any technique to induce the absence of sensation in a specific part of the body, generally for the aim of inducing local analgesia, that is, local insensitivity to pain, although other local senses may be affected as well. It ...
, is highly effective.
* Liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wid ...
cryosurgery
Cryosurgery is the use of extreme cold in surgery to destroy abnormal or diseased tissue; thus, it is the surgical application of cryoablation. The term comes from the Greek words cryo (κρύο) ("icy cold") and surgery (''cheirourgiki'' – χ� ...
is usually performed in an office visit, at weekly intervals. It is effective, inexpensive, safe for pregnancy, and does not usually cause scarring.
* Electrocauterization
Cauterization (or cauterisation, or cautery) is a medical practice or technique of burning a part of a body to remove or close off a part of it. It destroys some tissue in an attempt to mitigate bleeding and damage, remove an undesired growth, or ...
(sometimes called "loop electrical excision procedure" or LEEP) is a procedure with a long history of use and is considered effective.
* Laser ablation has less evidence to suggest its use. It may be less effective than other ablative methods. It is extremely expensive, and often used as a last resort.
* Formal surgical procedures, performed by a specialist under general anesthesia or spinal anesthesia
Spinal anaesthesia (or spinal anesthesia), also called spinal block, subarachnoid block, intradural block and intrathecal block, is a form of neuraxial regional anaesthesia involving the injection of a local anaesthetic or opioid into the subara ...
may be necessary for larger or more extensive warts, intra-anal warts, or warts in children. It carries a greater risk of scarring than other methods.
Topical agents
* A 0.15–0.5%podophyllotoxin
Podophyllotoxin (PPT) is the active ingredient in Podofilox, which is a medical cream that is used to treat genital warts and molluscum contagiosum. It is not recommended in HPV infections without external warts. It can be applied either by a he ...
(also called podofilox) solution in a gel or cream. It can be applied by the patient to the affected area and is not washed off. It is the purified and standardized active ingredient of podophyllin (see below). Podofilox is safer and more effective than podophyllin. Skin erosion and pain are more commonly reported than with imiquimod and sinecatechins. Its use is cycled (2 times per day for 3 days then 4–7 days off); one review states that it should only be used for four cycles.
* Imiquimod is a topical immune response cream, applied to the affected area. It causes less local irritation than podofilox but may cause fungal infections (11% in package insert) and flu-like symptoms (less than 5% disclosed in package insert).
* Sinecatechins is an ointment of catechin
Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
The name of the catechin chemical family derives from ''catechu'', which is the tanni ...
s (55% epigallocatechin gallate
Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is the ester of epigallocatechin and gallic acid, and is a type of catechin.
EGCG – the most abundant catechin in tea – is a polyphenol under basic research for i ...
) extracted from green tea
Green tea is a type of tea that is made from '' Camellia sinensis'' leaves and buds that have not undergone the same withering and oxidation process which is used to make oolong teas and black teas. Green tea originated in China, and since th ...
and other components. Mode of action is undetermined. It appears to have higher clearance rates than podophyllotoxin and imiquimod and causes less local irritation, but clearance takes longer than with imiquimod.
* Trichloroacetic acid (TCA) is less effective than cryosurgery
Cryosurgery is the use of extreme cold in surgery to destroy abnormal or diseased tissue; thus, it is the surgical application of cryoablation. The term comes from the Greek words cryo (κρύο) ("icy cold") and surgery (''cheirourgiki'' – χ� ...
, and is not recommended for use in the vagina, cervix, or urinary meatus.
* Interferon
Interferons (IFNs, ) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten th ...
can be used; it is effective, but it is also expensive and its effect is inconsistent.
Discontinued
* A 5% 5-fluorouracil
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, panc ...
(5-FU) cream was used, but it is no longer considered an acceptable treatment due to the side-effects.
Podophyllin, podofilox and isotretinoin
Isotretinoin, also known as 13-''cis''-retinoic acid and sold under the brand name Accutane among others, is a medication primarily used to treat severe acne. It is also used to prevent certain skin cancers (squamous-cell carcinoma), and in th ...
should not be used during pregnancy
Pregnancy is the time during which one or more offspring develops ( gestates) inside a woman's uterus (womb). A multiple pregnancy involves more than one offspring, such as with twins.
Pregnancy usually occurs by sexual intercourse, but ...
, as they could cause birth defect
A birth defect, also known as a congenital disorder, is an abnormal condition that is present at birth regardless of its cause. Birth defects may result in disabilities that may be physical, intellectual, or developmental. The disabilities ca ...
s in the fetus
A fetus or foetus (; plural fetuses, feti, foetuses, or foeti) is the unborn offspring that develops from an animal embryo. Following embryonic development the fetal stage of development takes place. In human prenatal development, fetal dev ...
.
Oral agents
Isotretinoin
Isotretinoin, also known as 13-''cis''-retinoic acid and sold under the brand name Accutane among others, is a medication primarily used to treat severe acne. It is also used to prevent certain skin cancers (squamous-cell carcinoma), and in th ...
taken orally has been shown to treat recalcitrant condylomata acuminata (RCA) of the cervix.
Epidemiology
Genital HPV infections have an estimated prevalence in the US of 10–20% and clinical manifestations in 1% of the sexually active adult population. US incidence of HPV infection has increased between 1975 and 2006. About 80% of those infected are between the ages of 17–33. Although treatments can remove warts, they do not remove the HPV, so warts can recur after treatment (about 50–73% of the timeCDC. (2004)REPORT TO CONGRESS: Prevention of Genital Human Papillomavirus Infection
). Warts can also spontaneously regress (with or without treatment). Traditional theories postulated that the virus remained in the body for a lifetime. However, studies using sensitive DNA techniques have shown that through immunological response, the virus can either be cleared or suppressed to levels below what
polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) ...
(PCR) tests can measure. One study testing genital skin for subclinical HPV using PCR found a prevalence of 10%.
Etymology
A ''condyloma acuminatum'' is a single genital wart, and ''condylomata acuminata'' are multiple genital warts. The word roots mean 'pointed wart' (from Greek κόνδυλος 'knuckle', Greek -ωμα '' -oma'' 'disease', and Latin '' acuminatum'' 'pointed'). Although similarly named, it is not the same as condyloma latum, which is a complication of secondarysyphilis
Syphilis () is a sexually transmitted infection caused by the bacterium '' Treponema pallidum'' subspecies ''pallidum''. The signs and symptoms of syphilis vary depending in which of the four stages it presents (primary, secondary, latent, a ...
.
References
External links
* {{DEFAULTSORT:Genital Wart Infections with a predominantly sexual mode of transmission Papillomavirus-associated diseases Virus-related cutaneous conditions Infectious causes of cancer Wikipedia medicine articles ready to translate