Gaseous water on:
[Wikipedia]
[Google]
[Amazon]
(99.9839 °C)
, -
, Boiling point
,
, -
,
 Water vapor will only condense onto another surface when that surface is cooler than the
Water vapor will only condense onto another surface when that surface is cooler than the
 Water vapor and dry air density calculations at 0 °C:
* The
Water vapor and dry air density calculations at 0 °C:
* The
 Gaseous water represents a small but environmentally significant constituent of the atmosphere. The percentage of water vapor in surface air varies from 0.01% at -42 °C (-44 °F) to 4.24% when the dew point is 30 °C (86 °F). Over 99% of atmospheric water is in the form of vapour, rather than liquid water or ice, and approximately 99.13% of the water vapour is contained in the
Gaseous water represents a small but environmentally significant constituent of the atmosphere. The percentage of water vapor in surface air varies from 0.01% at -42 °C (-44 °F) to 4.24% when the dew point is 30 °C (86 °F). Over 99% of atmospheric water is in the form of vapour, rather than liquid water or ice, and approximately 99.13% of the water vapour is contained in the
/ref>
/ref> this has likely happened, possibly more than once. Scientists thus distinguish between non-condensable (driving) and condensable (driven) greenhouse gases, i.e., the above water vapor feedback.Lacis, A. et al., The role of long-lived greenhouse gases as principal LW control knob that governs the global surface temperature for past and future climate change, Tellus B, vol. 65 p. 19734, 2013 Fog and clouds form through condensation around cloud condensation nuclei. In the absence of nuclei, condensation will only occur at much lower temperatures. Under persistent condensation or deposition, cloud droplets or snowflakes form, which
''Atmospheric Science: An Introductory Survey''
. Elsevier. Second Edition, 2006. . Page 8. and can be measured with a combination of land observations, weather balloons and satellites. The water content of the atmosphere as a whole is constantly depleted by precipitation. At the same time it is constantly replenished by evaporation, most prominently from oceans, lakes, rivers, and moist earth. Other sources of atmospheric water include combustion, respiration, volcanic eruptions, the transpiration of plants, and various other biological and geological processes. At any given time there is about 1.29 x 1016 litres (3.4 x 1015 gal.) of water in the atmosphere. The atmosphere holds 1 part in 2500 of the fresh water, and 1 part in 100,000 of the total water on Earth. The mean global content of water vapor in the atmosphere is roughly sufficient to cover the surface of the planet with a layer of liquid water about 25 mm deep. The mean annual precipitation for the planet is about 1 metre, a comparison which implies a rapid turnover of water in the air – on average, the residence time of a water molecule in the

 Geological formations such as cryogeysers are thought to exist on the surface of several icy moons ejecting water vapor due to tidal heating and may indicate the presence of substantial quantities of subsurface water. Plumes of water vapor have been detected on Jupiter's moon Europa (moon), Europa and are similar to plumes of water vapor detected on Saturn's moon Enceladus. Traces of water vapor have also been detected in the stratosphere of Titan (moon), Titan. Water vapor has been found to be a major constituent of the atmosphere of dwarf planet, Ceres (dwarf planet), Ceres, largest object in the asteroid belt The detection was made by using the Far-infrared astronomy, far-infrared abilities of the Herschel Space Observatory. The finding is unexpected because comets, not asteroids, are typically considered to "sprout jets and plumes." According to one of the scientists, "The lines are becoming more and more blurred between comets and asteroids." Scientists studying Mars hypothesize that if water moves about the planet, it does so as vapor.
The brilliance of
Geological formations such as cryogeysers are thought to exist on the surface of several icy moons ejecting water vapor due to tidal heating and may indicate the presence of substantial quantities of subsurface water. Plumes of water vapor have been detected on Jupiter's moon Europa (moon), Europa and are similar to plumes of water vapor detected on Saturn's moon Enceladus. Traces of water vapor have also been detected in the stratosphere of Titan (moon), Titan. Water vapor has been found to be a major constituent of the atmosphere of dwarf planet, Ceres (dwarf planet), Ceres, largest object in the asteroid belt The detection was made by using the Far-infrared astronomy, far-infrared abilities of the Herschel Space Observatory. The finding is unexpected because comets, not asteroids, are typically considered to "sprout jets and plumes." According to one of the scientists, "The lines are becoming more and more blurred between comets and asteroids." Scientists studying Mars hypothesize that if water moves about the planet, it does so as vapor.
The brilliance of
National Science Digital Library – Water Vapor
Calculate the condensation of your exhaled breath
Water Vapor Myths: A Brief Tutorial
– PhyMetrix {{Authority control Greenhouse gases Atmospheric thermodynamics Forms of water Water in gas Psychrometrics Articles containing video clips
specific gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
eous phase of water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
. It is one state
State may refer to:
Arts, entertainment, and media Literature
* ''State Magazine'', a monthly magazine published by the U.S. Department of State
* ''The State'' (newspaper), a daily newspaper in Columbia, South Carolina, United States
* ''Our S ...
of water within the hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to change in shape. This ...
. Water vapor
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Her ...
can be produced from the evaporation or boiling of liquid water or from the sublimation of ice
Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaqu ...
. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
and triggers convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the conve ...
currents that can lead to clouds.
Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
, where it acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
and methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
. Use of water vapor, as steam, has been important for cooking, and as a major component in energy production and transport systems since the industrial revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
.
Water vapor is a relatively common atmospheric constituent, present even in the solar atmosphere
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
as well as every planet in the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
and many astronomical objects including natural satellite
A natural satellite is, in the most common usage, an astronomical body that orbits a planet, dwarf planet, or small Solar System body (or sometimes another natural satellite). Natural satellites are often colloquially referred to as ''moons'' ...
s, comet
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena ...
s and even large asteroids. Likewise the detection of extrasolar water vapor would indicate a similar distribution in other planetary systems. Water vapor can also be indirect evidence supporting the presence of extraterrestrial liquid water in the case of some planetary mass objects.
Properties
Evaporation
Whenever a water molecule leaves a surface and diffuses into a surrounding gas, it is said to haveevaporated
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humi ...
. Each individual water molecule which transitions between a more associated (liquid) and a less associated (vapor/gas) state does so through the absorption or release of kinetic energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion.
It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acc ...
. The aggregate measurement of this kinetic energy transfer is defined as thermal energy and occurs only when there is differential in the temperature of the water molecules. Liquid water that becomes water vapor takes a parcel of heat with it, in a process called evaporative cooling
An evaporative cooler (also known as evaporative air conditioner, swamp cooler, swamp box, desert cooler and wet air cooler) is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning s ...
. The amount of water vapor in the air determines how frequently molecules will return to the surface. When a net evaporation occurs, the body of water will undergo a net cooling directly related to the loss of water.
In the US, the National Weather Service measures the actual rate of evaporation from a standardized "pan" open water surface outdoors, at various locations nationwide. Others do likewise around the world. The US data is collected and compiled into an annual evaporation map. The measurements range from under 30 to over 120 inches per year. Formulas can be used for calculating the rate of evaporation from a water surface such as a swimming pool. In some countries, the evaporation rate far exceeds the precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
rate.
Evaporative cooling is restricted by atmospheric conditions. Humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Humidity dep ...
is the amount of water vapor in the air. The vapor content of air is measured with devices known as hygrometer
A hair tension dial hygrometer with a nonlinear scale.
A hygrometer is an instrument used to measure the amount of water vapor in air, in soil, or in confined spaces. Humidity measurement instruments usually rely on measurements of some other qu ...
s. The measurements are usually expressed as specific humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Humidity depe ...
or percent relative humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Humidity dep ...
. The temperatures of the atmosphere and the water surface determine the equilibrium vapor pressure; 100% relative humidity occurs when the partial pressure of water vapor is equal to the equilibrium vapor pressure. This condition is often referred to as complete saturation. Humidity ranges from 0 grams per cubic metre in dry air to 30 grams per cubic metre (0.03 ounce per cubic foot) when the vapor is saturated at 30 °C.
Sublimation
Sublimation is the process by which water molecules directly leave the surface of ice without first becoming liquid water. Sublimation accounts for the slow mid-winter disappearance of ice and snow at temperatures too low to cause melting.Antarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean, it contains the geographic South Pole. Antarctica is the fifth-largest cont ...
shows this effect to a unique degree because it is by far the continent with the lowest rate of precipitation on Earth. As a result, there are large areas where millennial
Millennials, also known as Generation Y or Gen Y, are the Western demographic cohort following Generation X and preceding Generation Z. Researchers and popular media use the early 1980s as starting birth years and the mid-1990s to early 2000s ...
layers of snow have sublimed, leaving behind whatever non-volatile materials they had contained. This is extremely valuable to certain scientific disciplines, a dramatic example being the collection of meteorites that are left exposed in unparalleled numbers and excellent states of preservation.
Sublimation is important in the preparation of certain classes of biological specimens for scanning electron microscopy. Typically the specimens are prepared by cryofixation Cryofixation is a technique for fixation or stabilisation of biological materials as the first step in specimen preparation for electron microscopy and cryo-electron microscopy. Typical specimens for cryofixation include small samples of plant or an ...
and freeze-fracture, after which the broken surface is freeze-etched, being eroded by exposure to vacuum till it shows the required level of detail. This technique can display protein molecules, organelle structures and lipid bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many vir ...
s with very low degrees of distortion.
Condensation
 Water vapor will only condense onto another surface when that surface is cooler than the
Water vapor will only condense onto another surface when that surface is cooler than the dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
temperature, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a net warming occurs on that surface. The water molecule brings heat energy with it. In turn, the temperature of the atmosphere drops slightly. In the atmosphere, condensation produces clouds, fog and precipitation (usually only when facilitated by cloud condensation nuclei). The dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
of an air parcel is the temperature to which it must cool before water vapor in the air begins to condense. Condensation in the atmosphere forms cloud droplets.
Also, a net condensation of water vapor occurs on surfaces when the temperature of the surface is at or below the dew point temperature of the atmosphere. Deposition is a phase transition separate from condensation which leads to the direct formation of ice from water vapor. Frost and snow are examples of deposition.
There are several mechanisms of cooling by which condensation occurs:
1) Direct loss of heat by conduction or radiation.
2) Cooling from the drop in air pressure which occurs with uplift of air, also known as adiabatic cooling
In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, a ...
.
Air can be lifted by mountains, which deflect the air upward, by convection, and by cold and warm fronts.
3) Advective cooling - cooling due to horizontal movement of air.
Importance and Uses
* Provides water for plants and animals: Water vapour gets converted to rain and snow that serve as a natural source of water for plants and animals. * Controls evaporation: Excess water vapor in the air decreases the rate of evaporation. * Determines climatic conditions: Excess water vapor in the air produces rain, fog, snow etc. Hence, it determines climatic conditions.Chemical reactions
A number of chemical reactions have water as a product. If the reactions take place at temperatures higher than the dew point of the surrounding air the water will be formed as vapor and increase the local humidity, if below the dew point local condensation will occur. Typical reactions that result in water formation are the burning ofhydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
or hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s in air or other oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
containing gas mixtures, or as a result of reactions with oxidizers.
In a similar fashion other chemical or physical reactions can take place in the presence of water vapor resulting in new chemicals forming such as rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO( ...
on iron or steel, polymerization occurring (certain polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from ...
foams and cyanoacrylate glues cure with exposure to atmospheric humidity) or forms changing such as where anhydrous chemicals may absorb enough vapor to form a crystalline structure or alter an existing one, sometimes resulting in characteristic color changes that can be used for measurement.
Measurement
Measuring the quantity of water vapor in a medium can be done directly or remotely with varying degrees of accuracy. Remote methods such electromagnetic absorption are possible from satellites above planetary atmospheres. Direct methods may use electronic transducers, moistened thermometers or hygroscopic materials measuring changes in physical properties or dimensions.Impact on air density
Water vapor is lighter or less dense than dry air. At equivalent temperatures it is buoyant with respect to dry air, whereby the density of dry air at standard temperature and pressure (273.15 K, 101.325 kPa) is 1.27 g/L and water vapor at standard temperature has avapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phas ...
of 0.6 kPa and the much lower density of 0.0048 g/L.
Calculations
 Water vapor and dry air density calculations at 0 °C:
* The
Water vapor and dry air density calculations at 0 °C:
* The molar mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, ...
of water is , as calculated from the sum of the atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&nb ...
es of its constituent atoms.
* The average molar mass of air (approx. 78% nitrogen, N2; 21% oxygen, O2; 1% other gases) is at standard temperature and pressure (STP
STP may refer to:
Places
* São Tomé and Príncipe (ISO 3166-1 alpha-3 code, IOC country code, and FIFA country code STP)
* St Pancras railway station, London St Pancras (Domestic) railway station (National Rail code STP)
* St. Paul Downtown Air ...
).
* Obeying Avogadro's Law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific c ...
and the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
, moist air will have a lower density than dry air. At max. saturation (i. e. rel. humidity = 100% at 0 °C) the density will go down to 28.51 g/mol.
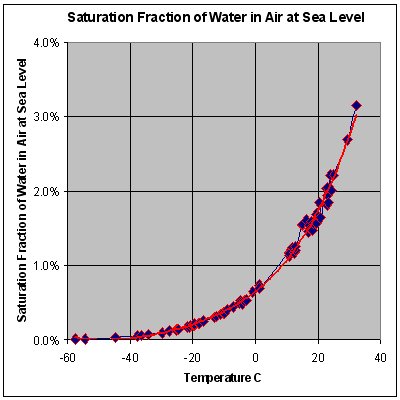
* STP conditions imply a temperature of 0 °C, at which the ability of water to become vapor is very restricted. Its concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', ...
in air is very low at 0 °C. The red line on the chart to the right is the maximum concentration of water vapor expected for a given temperature. The water vapor concentration increases significantly as the temperature rises, approaching 100% ( steam, pure water vapor) at 100 °C. However the difference in densities between air and water vapor would still exist (0.598 vs. 1.27 g/L).
At equal temperatures
At the same temperature, a column of dry air will be denser or heavier than a column of air containing any water vapor, the molar mass of diatomicnitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and diatomic oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
both being greater than the molar mass of water. Thus, any volume of dry air will sink if placed in a larger volume of moist air. Also, a volume of moist air will rise or be buoyant
Buoyancy (), or upthrust, is an upward force exerted by a fluid that opposes the weight of a partially or fully immersed object. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus the pr ...
if placed in a larger region of dry air. As the temperature rises the proportion of water vapor in the air increases, and its buoyancy will increase. The increase in buoyancy can have a significant atmospheric impact, giving rise to powerful, moisture rich, upward air currents when the air temperature and sea temperature reaches 25 °C or above. This phenomenon provides a significant driving force for cyclonic
In meteorology, a cyclone () is a large air mass that rotates around a strong center of low atmospheric pressure, counterclockwise in the Northern Hemisphere and clockwise in the Southern Hemisphere as viewed from above (opposite to an anti ...
and anticyclonic
An anticyclone is a weather phenomenon defined as a large-scale circulation of winds around a central region of high atmospheric pressure, clockwise in the Northern Hemisphere and counterclockwise in the Southern Hemisphere as viewed from abov ...
weather systems (typhoons and hurricanes).
Respiration and breathing
Water vapor is a by-product ofrespiration
Respiration may refer to:
Biology
* Cellular respiration, the process in which nutrients are converted into useful energy in a cell
** Anaerobic respiration, cellular respiration without oxygen
** Maintenance respiration, the amount of cellul ...
in plants and animals. Its contribution to the pressure, increases as its concentration increases. Its partial pressure contribution to air pressure increases, lowering the partial pressure contribution of the other atmospheric gases (Dalton's Law). The total air pressure must remain constant. The presence of water vapor in the air naturally dilutes or displaces the other air components as its concentration increases.
This can have an effect on respiration. In very warm air (35 °C) the proportion of water vapor is large enough to give rise to the stuffiness that can be experienced in humid jungle conditions or in poorly ventilated buildings.
Lifting gas
Water vapor has lower density than that ofair
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
and is therefore buoyant
Buoyancy (), or upthrust, is an upward force exerted by a fluid that opposes the weight of a partially or fully immersed object. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus the pr ...
in air but has lower vapor pressure than that of air. When water vapor is used as a lifting gas
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include ballo ...
by a thermal airship the water vapor is heated to form steam so that its vapor pressure is greater than the surrounding air pressure in order to maintain the shape of a theoretical "steam balloon", which yields approximately 60% the lift of helium and twice that of hot air.
General discussion
The amount of water vapor in an atmosphere is constrained by the restrictions of partial pressures and temperature. Dew point temperature and relative humidity act as guidelines for the process of water vapor in thewater cycle
The water cycle, also known as the hydrologic cycle or the hydrological cycle, is a biogeochemical cycle that describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly cons ...
. Energy input, such as sunlight, can trigger more evaporation on an ocean surface or more sublimation on a chunk of ice on top of a mountain. The ''balance'' between condensation and evaporation gives the quantity called vapor partial pressure.
The maximum partial pressure (''saturation pressure'') of water vapor in air varies with temperature of the air and water vapor mixture. A variety of empirical formulas exist for this quantity; the most used reference formula is the Goff-Gratch equation for the SVP over liquid water below zero degrees Celsius:
:
where , temperature of the moist air, is given in units of kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phy ...
, and is given in units of millibar
The bar is a metric unit of pressure, but not part of the International System of Units (SI). It is defined as exactly equal to 100,000 Pa (100 kPa), or slightly less than the current average atmospheric pressure on Earth at sea leve ...
s ( hectopascals).
The formula is valid from about −50 to 102 °C; however there are a very limited number of measurements of the vapor pressure of water over supercooled liquid water. There are a number of other formulae which can be used.
Under certain conditions, such as when the boiling temperature of water is reached, a net evaporation will always occur during standard atmospheric conditions regardless of the percent of relative humidity. This immediate process will dispel massive amounts of water vapor into a cooler atmosphere.
Exhaled air is almost fully at equilibrium with water vapor at the body temperature. In the cold air the exhaled vapor quickly condenses, thus showing up as a fog or mist of water droplets and as condensation or frost on surfaces. Forcibly condensing these water droplets from exhaled breath is the basis of exhaled breath condensate
Exhaled breath condensate (EBC) is the exhalate from breath, that has been condensed, typically via cooling using a collection device (commonly to 4 °C or subzero temperatures using a refrigerating device). EBC reflects changes in the respira ...
, an evolving medical diagnostic test.
Controlling water vapor in air is a key concern in the heating, ventilating, and air-conditioning (HVAC) industry. Thermal comfort
Thermal comfort is the condition of mind that expresses satisfaction with the thermal environment and is assessed by subjective evaluation ( ANSI/ASHRAE Standard 55).ANSI/ASHRAE Standard 55-2017, Thermal Environmental Conditions for Human Occupan ...
depends on the moist air conditions. Non-human comfort situations are called refrigeration
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, ht ...
, and also are affected by water vapor. For example, many food stores, like supermarkets, utilize open chiller cabinets, or ''food cases'', which can significantly lower the water vapor pressure (lowering humidity). This practice delivers several benefits as well as problems.
In Earth's atmosphere
 Gaseous water represents a small but environmentally significant constituent of the atmosphere. The percentage of water vapor in surface air varies from 0.01% at -42 °C (-44 °F) to 4.24% when the dew point is 30 °C (86 °F). Over 99% of atmospheric water is in the form of vapour, rather than liquid water or ice, and approximately 99.13% of the water vapour is contained in the
Gaseous water represents a small but environmentally significant constituent of the atmosphere. The percentage of water vapor in surface air varies from 0.01% at -42 °C (-44 °F) to 4.24% when the dew point is 30 °C (86 °F). Over 99% of atmospheric water is in the form of vapour, rather than liquid water or ice, and approximately 99.13% of the water vapour is contained in the troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
. The condensation of water vapor to the liquid or ice phase is responsible for clouds, rain, snow, and other precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
, all of which count among the most significant elements of what we experience as weather. Less obviously, the latent heat of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
, which is released to the atmosphere whenever condensation occurs, is one of the most important terms in the atmospheric energy budget on both local and global scales. For example, latent heat release in atmospheric convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the conve ...
is directly responsible for powering destructive storms such as tropical cyclones
A tropical cyclone is a rapidly rotating storm system characterized by a low-pressure center, a closed low-level atmospheric circulation, strong winds, and a spiral arrangement of thunderstorms that produce heavy rain and squalls. Dependi ...
and severe thunderstorms
A thunderstorm, also known as an electrical storm or a lightning storm, is a storm characterized by the presence of lightning and its acoustic effect on the Earth's atmosphere, known as thunder. Relatively weak thunderstorms are somet ...
. Water vapor is an important greenhouse gas owing to the presence of the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
bond which strongly absorbs in the infra-red.
Water vapor is the "working medium" of the atmospheric thermodynamic engine which transforms heat energy from sun irradiation into mechanical energy in the form of winds. Transforming thermal energy into mechanical energy requires an upper and a lower temperature level, as well as a working medium which shuttles forth and back between both. The upper temperature level is given by the soil or water surface of the earth, which absorbs the incoming sun radiation and warms up, evaporating water. The moist and warm air at the ground is lighter than its surroundings and rises up to the upper limit of the troposphere. There the water molecules radiate their thermal energy into outer space, cooling down the surrounding air. The upper atmosphere constitutes the lower temperature level of the atmospheric thermodynamic engine. The water vapor in the now cold air condenses out and falls down to the ground in the form of rain or snow. The now heavier cold and dry air sinks down to ground as well; the atmospheric thermodynamic engine thus establishes a vertical convection, which transports heat from the ground into the upper atmosphere, where the water molecules can radiate it to outer space. Due to the earth's rotation and the resulting Coriolis forces, this vertical atmospheric convection is also converted into a horizontal convection, in the form of cyclones and anticyclones, which transport the water evaporated over the oceans into the interior of the continents, enabling vegetation to grow.
Water in Earth's atmosphere is not merely below its boiling point (100 °C), but at altitude it goes below its freezing point (0 °C), due to water's highly polar attraction. When combined with its quantity, water vapor then has a relevant dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
and frost point, unlike e. g., carbon dioxide and methane. Water vapor thus has a scale height
In atmospheric, earth, and planetary sciences, a scale height, usually denoted by the capital letter ''H'', is a distance ( vertical or radial) over which a physical quantity decreases by a factor of e (the base of natural logarithms, approxima ...
a fraction of that of the bulk atmosphere, as the water condenses
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
and exits, primarily in the troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
, the lowest layer of the atmosphere. Carbon dioxide () and methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
, being well-mixed in the atmosphere, tend to rise above water vapour. The absorption and emission of both compounds contribute to Earth's emission to space, and thus the planetary greenhouse effect. This greenhouse forcing is directly observable, via distinct spectral features versus water vapor, and observed to be rising with rising levels. Conversely, adding water vapor at high altitudes has a disproportionate impact, which is why jet traffic has a disproportionately high warming effect. Oxidation of methane is also a major source of water vapour in the stratosphere, and adds about 15% to methane's global warming effect.
In the absence of other greenhouse gases, Earth's water vapor would condense to the surface;What is the maximum and minimum distance for the Earth that is compatible with life?/ref>
/ref> this has likely happened, possibly more than once. Scientists thus distinguish between non-condensable (driving) and condensable (driven) greenhouse gases, i.e., the above water vapor feedback.Lacis, A. et al., The role of long-lived greenhouse gases as principal LW control knob that governs the global surface temperature for past and future climate change, Tellus B, vol. 65 p. 19734, 2013 Fog and clouds form through condensation around cloud condensation nuclei. In the absence of nuclei, condensation will only occur at much lower temperatures. Under persistent condensation or deposition, cloud droplets or snowflakes form, which
precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
when they reach a critical mass.
Atmospheric concentration of water vapour is highly variable between locations and times, from 10 ppmv in the coldest air to 5% (50 000 ppmv) in humid tropical air,Wallace, John M. and Peter V. Hobbs''Atmospheric Science: An Introductory Survey''
. Elsevier. Second Edition, 2006. . Page 8. and can be measured with a combination of land observations, weather balloons and satellites. The water content of the atmosphere as a whole is constantly depleted by precipitation. At the same time it is constantly replenished by evaporation, most prominently from oceans, lakes, rivers, and moist earth. Other sources of atmospheric water include combustion, respiration, volcanic eruptions, the transpiration of plants, and various other biological and geological processes. At any given time there is about 1.29 x 1016 litres (3.4 x 1015 gal.) of water in the atmosphere. The atmosphere holds 1 part in 2500 of the fresh water, and 1 part in 100,000 of the total water on Earth. The mean global content of water vapor in the atmosphere is roughly sufficient to cover the surface of the planet with a layer of liquid water about 25 mm deep. The mean annual precipitation for the planet is about 1 metre, a comparison which implies a rapid turnover of water in the air – on average, the residence time of a water molecule in the
troposphere
The troposphere is the first and lowest layer of the atmosphere of the Earth, and contains 75% of the total mass of the planetary atmosphere, 99% of the total mass of water vapour and aerosols, and is where most weather phenomena occur. From ...
is about 9 to 10 days.
Global mean water vapour is about 0.25% of the atmosphere by mass and also varies seasonally, in terms of contribution to atmospheric pressure between 2.62 hPa in July and 2.33 hPa in December. IPCC AR6 expresses medium confidence in increase of total water vapour at about 1-2% per decade; it is expected to increase by around 7% per °C of warming.
Episodes of surface geothermal activity, such as volcanic eruptions and geysers, release variable amounts of water vapor into the atmosphere. Such eruptions may be large in human terms, and major explosive eruptions may inject exceptionally large masses of water exceptionally high into the atmosphere, but as a percentage of total atmospheric water, the role of such processes is trivial. The relative concentrations of the various gases emitted by volcano
A volcano is a rupture in the Crust (geology), crust of a Planet#Planetary-mass objects, planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and volcanic gas, gases to escape from a magma chamber below the surface.
On Ear ...
es varies considerably according to the site and according to the particular event at any one site. However, water vapor is consistently the commonest volcanic gas
Volcanic gases are gases given off by active (or, at times, by dormant) volcanoes. These include gases trapped in cavities (vesicles) in volcanic rocks, dissolved or dissociated gases in magma and lava, or gases emanating from lava, from volcani ...
; as a rule, it comprises more than 60% of total emissions during a subaerial eruption A subaerial eruption is any sort of volcanic eruption that occurs on the Earth's surface, or in the open air 'under the air', and not underwater or underground. They generally produce pyroclastic flows, lava fountains, and lava flows, which are com ...
.
Atmospheric water vapor content is expressed using various measures. These include vapor pressure, specific humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Humidity depe ...
, mixing ratio, dew point temperature, and relative humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Humidity dep ...
.
Radar and satellite imaging
Because water molecules absorbmicrowave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ra ...
s and other radio wave frequencies, water in the atmosphere attenuates radar
Radar is a detection system that uses radio waves to determine the distance ('' ranging''), angle, and radial velocity of objects relative to the site. It can be used to detect aircraft, ships, spacecraft, guided missiles, motor vehicles, we ...
signals. In addition, atmospheric water will reflect and refract
In physics, refraction is the redirection of a wave as it passes from one medium to another. The redirection can be caused by the wave's change in speed or by a change in the medium. Refraction of light is the most commonly observed phenomeno ...
signals to an extent that depends on whether it is vapor, liquid or solid.
Generally, radar signals lose strength progressively the farther they travel through the troposphere. Different frequencies attenuate at different rates, such that some components of air are opaque to some frequencies and transparent to others. Radio waves used for broadcasting and other communication experience the same effect.
Water vapor reflects radar to a lesser extent than do water's other two phases. In the form of drops and ice crystals, water acts as a prism, which it does not do as an individual molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
; however, the existence of water vapor in the atmosphere causes the atmosphere to act as a giant prism.
A comparison of GOES-12
GOES-12, known as GOES-M before becoming operational, is an American weather satellite, which is part of the US National Oceanic and Atmospheric Administration's Geostationary Operational Environmental Satellite system. It was launched on July 23, ...
satellite images shows the distribution of atmospheric water vapor relative to the oceans, clouds and continents of the Earth. Vapor surrounds the planet but is unevenly distributed. The image loop on the right shows monthly average of water vapor content with the units are given in centimeters, which is the precipitable water Precipitable water is the depth of water in a column of the atmosphere, if all the water in that column were precipitated as rain. As a depth, the precipitable water is measured in millimeters or inches. Often abbreviated as "TPW", for Total Preci ...
or equivalent amount of water that could be produced if all the water vapor in the column were to condense. The lowest amounts of water vapor (0 centimeters) appear in yellow, and the highest amounts (6 centimeters) appear in dark blue. Areas of missing data appear in shades of gray. The maps are based on data collected by the Moderate Resolution Imaging Spectroradiometer
The Moderate Resolution Imaging Spectroradiometer (MODIS) is a satellite-based sensor used for earth and climate measurements. There are two MODIS sensors in Earth orbit: one on board the Terra ( EOS AM) satellite, launched by NASA in 19 ...
(MODIS) sensor on NASA's Aqua satellite. The most noticeable pattern in the time series is the influence of seasonal temperature changes and incoming sunlight on water vapor. In the tropics, a band of extremely humid air wobbles north and south of the equator as the seasons change. This band of humidity is part of the Intertropical Convergence Zone, where the easterly trade winds from each hemisphere converge and produce near-daily thunderstorms and clouds. Farther from the equator, water vapor concentrations are high in the hemisphere experiencing summer and low in the one experiencing winter. Another pattern that shows up in the time series is that water vapor amounts over land areas decrease more in winter months than adjacent ocean areas do. This is largely because air temperatures over land drop more in the winter than temperatures over the ocean. Water vapor condenses more rapidly in colder air.
As water vapor absorbs light in the visible spectral range, its absorption can be used in spectroscopic applications (such as DOAS
doas (“dedicated openbsd application subexecutor”) is a program to execute commands as another user. The system administrator can configure it to give specified users privileges to execute specified commands. It is free and open-source unde ...
) to determine the amount of water vapor in the atmosphere. This is done operationally, e.g. from the Global Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
Monitoring Experiment (GOME) spectrometers on ERS (GOME) and MetOp (GOME-2). The weaker water vapor absorption lines in the blue spectral range and further into the UV up to its dissociation limit around 243 nm are mostly based on quantum mechanical calculations and are only partly confirmed by experiments.
Lightning generation
Water vapor plays a key role in lightning production in the atmosphere. From cloud physics, usually clouds are the real generators of static electric charge, charge as found in Earth's atmosphere. The ability of clouds to hold massive amounts of electrical energy is directly related to the amount of water vapor present in the local system. The amount of water vapor directly controls the permittivity of the air. During times of low humidity, static discharge is quick and easy. During times of higher humidity, fewer static discharges occur. Permittivity and capacitance work hand in hand to produce the megawatt outputs of lightning. After a cloud, for instance, has started its way to becoming a lightning generator, atmospheric water vapor acts as a substance (or electrical insulation, insulator) that decreases the ability of the cloud to electrostatic discharge, discharge its electrical energy. Over a certain amount of time, if the cloud continues to generate and store more static electricity, the barrier that was created by the atmospheric water vapor will ultimately break down from the stored electrical potential energy. This energy will be released to a local oppositely charged region, in the form of lightning. The strength of each discharge is directly related to the atmospheric permittivity, capacitance, and the source's charge generating ability.Extraterrestrial
Water vapor is common in theSolar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
and by extension, other planetary systems. Its signature has been detected in the atmospheres of the Sun, occurring in sunspots. The presence of water vapor has been detected in the atmospheres of all seven extraterrestrial planets in the solar system, the Earth's Moon, and the moons of other planets, although typically in only trace amounts.

 Geological formations such as cryogeysers are thought to exist on the surface of several icy moons ejecting water vapor due to tidal heating and may indicate the presence of substantial quantities of subsurface water. Plumes of water vapor have been detected on Jupiter's moon Europa (moon), Europa and are similar to plumes of water vapor detected on Saturn's moon Enceladus. Traces of water vapor have also been detected in the stratosphere of Titan (moon), Titan. Water vapor has been found to be a major constituent of the atmosphere of dwarf planet, Ceres (dwarf planet), Ceres, largest object in the asteroid belt The detection was made by using the Far-infrared astronomy, far-infrared abilities of the Herschel Space Observatory. The finding is unexpected because comets, not asteroids, are typically considered to "sprout jets and plumes." According to one of the scientists, "The lines are becoming more and more blurred between comets and asteroids." Scientists studying Mars hypothesize that if water moves about the planet, it does so as vapor.
The brilliance of
Geological formations such as cryogeysers are thought to exist on the surface of several icy moons ejecting water vapor due to tidal heating and may indicate the presence of substantial quantities of subsurface water. Plumes of water vapor have been detected on Jupiter's moon Europa (moon), Europa and are similar to plumes of water vapor detected on Saturn's moon Enceladus. Traces of water vapor have also been detected in the stratosphere of Titan (moon), Titan. Water vapor has been found to be a major constituent of the atmosphere of dwarf planet, Ceres (dwarf planet), Ceres, largest object in the asteroid belt The detection was made by using the Far-infrared astronomy, far-infrared abilities of the Herschel Space Observatory. The finding is unexpected because comets, not asteroids, are typically considered to "sprout jets and plumes." According to one of the scientists, "The lines are becoming more and more blurred between comets and asteroids." Scientists studying Mars hypothesize that if water moves about the planet, it does so as vapor.
The brilliance of comet
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena ...
tails comes largely from water vapor. On approach to the Sun, the ice many comets carry Sublimation (phase transition), sublimes to vapor. Knowing a comet's distance from the sun, astronomers may deduce the comet's water content from its brilliance.
Water vapor has also been confirmed outside the Solar System. Spectroscopic analysis of HD 209458 b, an extrasolar planet in the constellation Pegasus, provides the first evidence of atmospheric water vapor beyond the Solar System. A star called IRC +10216, CW Leonis was found to have a ring of vast quantities of water vapor circling the aging, massive star. A NASA satellite designed to study chemicals in interstellar gas clouds, made the discovery with an onboard spectrometer. Most likely, "the water vapor was vaporized from the surfaces of orbiting comets." Other exoplanets with evidence of water vapor include HAT-P-11b and K2-18b.
See also
* Air density * Atmospheric river * Boiling point * Condensation in aerosol dynamics * Deposition *Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
* Eddy covariance
* Equation of state
* Evaporative cooler
* Fog
* Frost
* Gas laws
* Gibbs free energy
* Gibbs phase rule
* Greenhouse gas
* Heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
* Heat of vaporization
* Humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Humidity dep ...
* Hygrometer
* Ideal gas
* Kinetic theory of gases
* Latent heat
* Latent heat flux
* Microwave radiometer
* Phase of matter
* Saturation vapor density
* Steam
* Sublimation
* Superheating
* Supersaturation
* Thermodynamics
* Troposphere
* Vapor pressure
References
Bibliography
* * * * * * * * * * *External links
National Science Digital Library – Water Vapor
Calculate the condensation of your exhaled breath
Water Vapor Myths: A Brief Tutorial
– PhyMetrix {{Authority control Greenhouse gases Atmospheric thermodynamics Forms of water Water in gas Psychrometrics Articles containing video clips