Gadolinia on:
[Wikipedia]
[Google]
[Amazon]
Gadolinium(III) oxide (archaically gadolinia) is an inorganic compound with the formula Gd2O3. It is one of the most commonly available forms of the

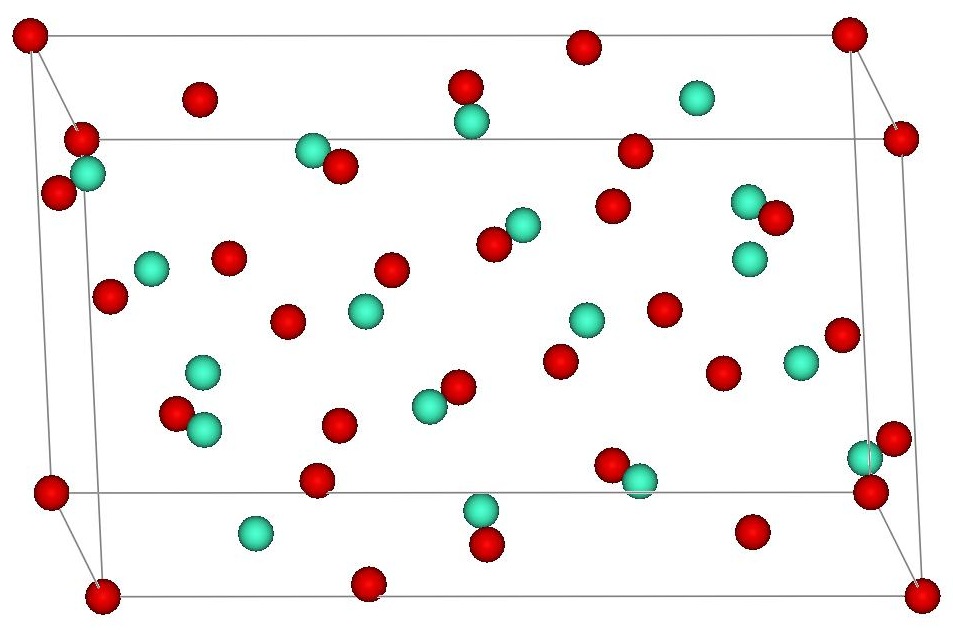 Gadolinium oxide adopts two structures. The cubic ( cI80, Ia), No. 206) structure is similar to that of manganese(III) oxide and heavy trivalent lanthanide sesquioxides. The cubic structure features two types of gadolinium sites, each with a coordination number of 6 but with different coordination geometries. The second polymorph is
Gadolinium oxide adopts two structures. The cubic ( cI80, Ia), No. 206) structure is similar to that of manganese(III) oxide and heavy trivalent lanthanide sesquioxides. The cubic structure features two types of gadolinium sites, each with a coordination number of 6 but with different coordination geometries. The second polymorph is
rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
, derivatives of which are potential contrast agents for magnetic resonance imaging.
Structure

 Gadolinium oxide adopts two structures. The cubic ( cI80, Ia), No. 206) structure is similar to that of manganese(III) oxide and heavy trivalent lanthanide sesquioxides. The cubic structure features two types of gadolinium sites, each with a coordination number of 6 but with different coordination geometries. The second polymorph is
Gadolinium oxide adopts two structures. The cubic ( cI80, Ia), No. 206) structure is similar to that of manganese(III) oxide and heavy trivalent lanthanide sesquioxides. The cubic structure features two types of gadolinium sites, each with a coordination number of 6 but with different coordination geometries. The second polymorph is monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic s ...
( Pearson symbol mS30, space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it uncha ...
C2/m, No. 12). At room temperature, the cubic structure is more stable. The phase change to the monoclinic structure takes place at 1200 °C. Above 2100 °C to the melting point at 2420 °C, a hexagonal phase dominates.
Preparation and chemistry
Gadolinium oxide can be formed by thermal decomposition of the hydroxide, nitrate, carbonate, or oxalates. Gadolinium oxide forms on the surface of gadolinium metal. Gadolinium oxide is a rather basic oxide, indicated by its ready reaction with carbon dioxide to give carbonates. It dissolves readily in the common mineral acids with the complication that theoxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
, fluoride, sulfate and phosphate are very insoluble in water and may coat the grains of oxide, thereby preventing the complete dissolution.Yost, D.M, Russell, H. Jr., Garner, C.S. ''The Rare-Earth Elements and their Compounds'', Wiley, 1947.
Nanoparticles of Gd2O3
Several methods are known for the synthesis of gadolinium oxidenanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
s, mostly based on precipitation of the hydroxide by the reaction of gadolinium ions with hydroxide, followed by thermal dehydration to the oxide. The nanoparticles are always coated with a protective material to avoid the formation of larger polycrystalline aggregates.
Nanoparticles of gadolinium oxide is a potential contrast agent for magnetic resonance imaging (MRI). A dextran
Dextran is a complex branched glucan ( polysaccharide derived from the condensation of glucose), originally derived from wine. IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 ...
-coated preparation of 20–40 nm sized gadolinium oxide particles had a relaxivity of 4.8 s−1mM−1 per gadolinium ion at 7.05 T (an unusually high field compared to the clinically used MRI scanners which mostly range from 0.5 to 3 T). Smaller particles, between 2 and 7 nm, were tested as an MRI agent.
References
{{Gadolinium compounds Gadolinium compounds Sesquioxides