Ytterbium(III) oxide.jpg on:
[Wikipedia]
[Google]
[Amazon]
Ytterbium is a
 Ytterbium is found with other
Ytterbium is found with other
 The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium,
The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium,
 Ytterbium forms both dihalides and trihalides with the halogens fluorine,
Ytterbium forms both dihalides and trihalides with the halogens fluorine,
p. 448
 Ytterbium was discovered by the Swiss chemist
Ytterbium was discovered by the Swiss chemist
It's Elemental – Ytterbium
*
Encyclopedia of Geochemistry - Ytterbium
{{Good article Chemical elements Chemical elements with face-centered cubic structure Lanthanides Suspected teratogens
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Yb and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. However, like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density and melting and boiling points differ significantly from those of most other lanthanides.
In 1878, the Swiss chemist Jean Charles Galissard de Marignac
Jean Charles Galissard de Marignac (24 April 1817 – 15 April 1894) was a Swiss chemist whose work with atomic weights suggested the possibility of isotopes and the packing fraction of nuclei. His study of the rare earth elements led to h ...
separated from the rare earth "erbia" another independent component, which he called " ytterbia", for Ytterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is per ...
, the village in Sweden near where he found the new component of erbium
Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, or ...
. He suspected that ytterbia was a compound of a new element that he called "ytterbium" (in total, four elements were named after the village, the others being yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
, terbium
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with w ...
, and erbium
Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, or ...
). In 1907, the new earth "lutecia" was separated from ytterbia, from which the element "lutecium" (now lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
) was extracted by Georges Urbain Georges Urbain (12 April 1872 – 5 November 1938) was a French chemist, a professor of the Sorbonne, a member of the Institut de France, and director of the Institute of Chemistry in Paris. Much of his work focused on the rare earths, isolating a ...
, Carl Auer von Welsbach
Carl Auer von Welsbach (1 September 1858 – 4 August 1929), who received the Austrian noble title of Freiherr Auer von Welsbach in 1901, was an Austrian scientist and inventor, who separated didymium into the elements neodymium and praseo ...
, and Charles James Charles James may refer to:
* Charles James (British Army officer) (1757/8–1821), English army officer and writer
* Charles James (attorney) (born 1954), former U.S. assistant attorney general
* Charles James (American football) (born 1990), Amer ...
. After some discussion, Marignac's name "ytterbium" was retained. A relatively pure sample of the metal was not obtained until 1953. At present, ytterbium is mainly used as a dopant
A dopant, also called a doping agent, is a trace of impurity element that is introduced into a chemical material to alter its original electrical or optical properties. The amount of dopant necessary to cause changes is typically very low. When ...
of stainless steel or active laser media, and less often as a gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
source.
Natural ytterbium is a mixture of seven stable isotopes, which altogether are present at concentrations of 0.3 parts per million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, th ...
. This element is mined in China, the United States, Brazil, and India in form of the minerals monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the ceriu ...
, euxenite, and xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
. The ytterbium concentration is low because it is found only among many other rare-earth elements
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silve ...
; moreover, it is among the least abundant. Once extracted and prepared, ytterbium is somewhat hazardous as an eye and skin irritant. The metal is a fire and explosion hazard.
Characteristics
Physical properties
Ytterbium is a soft,malleable
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
and ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
that displays a bright silvery luster when pure. It is a rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
, and it is readily dissolved by the strong mineral acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Cha ...
s. It reacts slowly with cold water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
and it oxidizes slowly in air.
Ytterbium has three allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
labeled by the Greek letters alpha, beta and gamma; their transformation temperatures are −13 ° C and 795 °C, although the exact transformation temperature depends on the pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
and stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
. The beta allotrope (6.966 g/cm3) exists at room temperature, and it has a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns ...
. The high-temperature gamma allotrope (6.57 g/cm3) has a body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
crystalline structure. The alpha allotrope (6.903 g/cm3) has a hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
crystalline structure and is stable at low temperatures. The beta allotrope has a metallic electrical conductivity at normal atmospheric pressure, but it becomes a semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
when exposed to a pressure of about 16,000 atmospheres (1.6 GPa
Grading in education is the process of applying standardized measurements for varying levels of achievements in a course. Grades can be assigned as letters (usually A through F), as a range (for example, 1 to 6), as a percentage, or as a numbe ...
). Its electrical resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
increases ten times upon compression to 39,000 atmospheres (3.9 GPa), but then drops to about 10% of its room-temperature resistivity at about 40,000 atm (4.0 GPa).
In contrast with the other rare-earth metals, which usually have antiferromagnetic
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
and/or ferromagnetic properties at low temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
s, ytterbium is paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
at temperatures above 1.0 kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and phy ...
. However, the alpha allotrope is diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
. With a melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
of 824 °C and a boiling point of 1196 °C, ytterbium has the smallest liquid range of all the metals.
Contrary to most other lanthanides, which have a close-packed hexagonal lattice, ytterbium crystallizes in the face-centered cubic system. Ytterbium has a density of 6.973 g/cm3, which is significantly lower than those of the neighboring lanthanides, thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ...
(9.32 g/cm3) and lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
(9.841 g/cm3). Its melting and boiling points are also significantly lower than those of thulium and lutetium. This is due to the closed-shell electron configuration of ytterbium ( e4f14 6s2), which causes only the two 6s electrons to be available for metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be des ...
ing (in contrast to the other lanthanides where three electrons are available) and increases ytterbium's metallic radius.
Chemical properties
Ytterbium metal tarnishes slowly in air, taking on a golden or brown hue. Finely dispersed ytterbium readily oxidizes in air and under oxygen. Mixtures of powdered ytterbium with polytetrafluoroethylene orhexachloroethane
Hexachloroethane, also known as perchloroethane is the organochlorine compound with the chemical formula . It is white solid at room temperature with a camphor-like odor. It has been used by the military in smoke compositions, such as base-eject ...
burn with a luminous emerald-green flame. Ytterbium reacts with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
to form various non-stoichiometric
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); m ...
hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s. Ytterbium dissolves slowly in water, but quickly in acids, liberating hydrogen gas.
Ytterbium is quite electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, and it reacts slowly with cold water and quite quickly with hot water to form ytterbium(III) hydroxide:
:2 Yb (s) + 6 H2O (l) → 2 Yb(OH)3 (aq) + 3 H2 (g)
Ytterbium reacts with all the halogens:
:2 Yb (s) + 3 F2 (g) → 2 YbF3 (s) hite Hite or HITE may refer to:
*HiteJinro, a South Korean brewery
**Hite Brewery
*Hite (surname)
*Hite, California, former name of Hite Cove, California
*Hite, Utah, a ghost town
* HITE, an industrial estate in Pakistan
See also
*''Hite v. Fairfax
...
:2 Yb (s) + 3 Cl2 (g) → 2 YbCl3 (s) hite Hite or HITE may refer to:
*HiteJinro, a South Korean brewery
**Hite Brewery
*Hite (surname)
*Hite, California, former name of Hite Cove, California
*Hite, Utah, a ghost town
* HITE, an industrial estate in Pakistan
See also
*''Hite v. Fairfax
...
:2 Yb (s) + 3 Br2 (g) → 2 YbBr3 (s) hite Hite or HITE may refer to:
*HiteJinro, a South Korean brewery
**Hite Brewery
*Hite (surname)
*Hite, California, former name of Hite Cove, California
*Hite, Utah, a ghost town
* HITE, an industrial estate in Pakistan
See also
*''Hite v. Fairfax
...
:2 Yb (s) + 3 I2 (g) → 2 YbI3 (s) hite Hite or HITE may refer to:
*HiteJinro, a South Korean brewery
**Hite Brewery
*Hite (surname)
*Hite, California, former name of Hite Cove, California
*Hite, Utah, a ghost town
* HITE, an industrial estate in Pakistan
See also
*''Hite v. Fairfax
...
The ytterbium(III) ion absorbs light in the near infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from arou ...
range of wavelengths, but not in visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
, so ytterbia, Yb2O3, is white in color and the salts of ytterbium are also colorless. Ytterbium dissolves readily in dilute sulfuric acid to form solutions that contain the colorless Yb(III) ions, which exist as nonahydrate complexes:
:2 Yb (s) + 3 H2SO4 (aq) + 18 (l) → 2 b(H2O)9sup>3+ (aq) + 3 (aq) + 3 H2 (g)
Yb(II) vs. Yb(III)
Although usually trivalent, ytterbium readily forms divalent compounds. This behavior is unusual for lanthanides, which almost exclusively form compounds with an oxidation state of +3. The +2 state has a valence electron configuration of 4''f''14 because the fully filled ''f''-shell gives more stability. The yellow-green ytterbium(II) ion is a very strongreducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth me ...
and decomposes water, releasing hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
gas, and thus only the colorless ytterbium(III) ion occurs in aqueous solution. Samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samar ...
and thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ...
also behave this way in the +2 state, but europium(II) is stable in aqueous solution. Ytterbium metal behaves similarly to europium metal and the alkaline earth metals, dissolving in ammonia to form blue electride
An electride is an ionic compound in which an electron is the anion. Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of a(NH3)6sup>+ and solvated electrons:
:Na + 6 NH3 → ...
salts.
Isotopes
Natural ytterbium is composed of seven stableisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
s: 168Yb, 170Yb, 171Yb, 172Yb, 173Yb, 174Yb, and 176Yb, with 174Yb being the most common, at 31.8% of the natural abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomi ...
). 27 radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s have been observed, with the most stable ones being 169Yb with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 32.0 days, 175Yb with a half-life of 4.18 days, and 166Yb with a half-life of 56.7 hours. All of the remaining radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
isotopes have half-lives that are less than two hours, and most of these have half-lives under 20 minutes. Ytterbium also has 12 meta state
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
s, with the most stable being 169mYb (''t''1/2 46 seconds).
The isotopes of ytterbium range in atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
from 147.9674 atomic mass unit
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at ...
(u) for 148Yb to 180.9562 u for 181Yb. The primary decay mode of ytterbium isotopes lighter than the most abundant stable isotope, 174Yb, is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Thi ...
, and the primary decay mode for those heavier than 174Yb is beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
. The primary decay products of ytterbium isotopes lighter than 174Yb are thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ...
isotopes, and the primary decay products of ytterbium isotopes with heavier than 174Yb are lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
isotopes.
Occurrence
 Ytterbium is found with other
Ytterbium is found with other rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
s in several rare mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
s. It is most often recovered commercially from monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the ceriu ...
sand (0.03% ytterbium). The element is also found in euxenite and xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
. The main mining areas are China, the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ...
, Brazil
Brazil ( pt, Brasil; ), officially the Federative Republic of Brazil (Portuguese: ), is the largest country in both South America and Latin America. At and with over 217 million people, Brazil is the world's fifth-largest country by area ...
, India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the seventh-largest country by area, the second-most populous country, and the most populous democracy in the world. Bounded by the Indian Ocean on the so ...
, Sri Lanka, and Australia. Reserves of ytterbium are estimated as one million tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s. Ytterbium is normally difficult to separate from other rare earths, but ion-exchange and solvent extraction
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
techniques developed in the mid- to late 20th century have simplified separation. Compounds of ytterbium are rare and have not yet been well characterized. The abundance of ytterbium in the Earth's crust is about 3 mg/kg.
As an even-numbered lanthanide, in accordance with the Oddo-Harkins rule, ytterbium is significantly more abundant than its immediate neighbors, thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ...
and lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
, which occur in the same concentrate at levels of about 0.5% each. The world production of ytterbium is only about 50 tonnes per year, reflecting that it has few commercial applications. Microscopic traces of ytterbium are used as a dopant
A dopant, also called a doping agent, is a trace of impurity element that is introduced into a chemical material to alter its original electrical or optical properties. The amount of dopant necessary to cause changes is typically very low. When ...
in the Yb:YAG laser, a solid-state laser
A solid-state laser is a laser that uses a gain medium that is a solid, rather than a liquid as in dye lasers or a gas as in gas lasers. Semiconductor-based lasers are also in the solid state, but are generally considered as a separate class ...
in which ytterbium is the element that undergoes stimulated emission of electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible) li ...
.
Ytterbium is often the most common substitute in yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
minerals. In very few known cases/occurrences ytterbium prevails over yttrium, as, e.g., in xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
-(Yb). A report of native ytterbium from the Moon's regolith is known.
Production
It is relatively difficult to separate ytterbium from other lanthanides due to its similar properties. As a result, the process is somewhat long. First, minerals such asmonazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the ceriu ...
or xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
are dissolved into various acids, such as sulfuric acid. Ytterbium can then be separated from other lanthanides by ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
, as can other lanthanides. The solution is then applied to a resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on n ...
, which different lanthanides bind in different matters. This is then dissolved using complexing agent
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
s, and due to the different types of bonding exhibited by the different lanthanides, it is possible to isolate the compounds.
Ytterbium is separated from other rare earths either by ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
or by reduction with sodium amalgam. In the latter method, a buffered acidic solution of trivalent rare earths is treated with molten sodium-mercury alloy, which reduces and dissolves Yb3+. The alloy is treated with hydrochloric acid. The metal is extracted from the solution as oxalate and converted to oxide by heating. The oxide is reduced to metal by heating with lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lant ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
or zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
in high vacuum. The metal is purified by sublimation and collected over a condensed plate.
Compounds
 The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium,
The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samar ...
, and thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ...
, the trihalides of ytterbium can be reduced to the dihalides by hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...
compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic ...
(CaO).
Halides
 Ytterbium forms both dihalides and trihalides with the halogens fluorine,
Ytterbium forms both dihalides and trihalides with the halogens fluorine, chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
, bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
, and iodine. The dihalides are susceptible to oxidation to the trihalides at room temperature and disproportionate to the trihalides and metallic ytterbium at high temperature:
:3 YbX2 → 2 YbX3 + Yb (X = F, Cl, Br, I)
Some ytterbium halides are used as reagents in organic synthesis. For example, ytterbium(III) chloride (YbCl3) is a Lewis acid and can be used as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
in the Aldol
In organic chemistry, an aldol describes a structural motif consisting of a 3-hydroxy ketone or 3-hydroxyaldehyde. Aldols are usually the product of aldol addition. When used alone, the term "aldol" may refer to 3-hydroxybutanal.
Stereochemistry
...
and Diels–Alder reactions. Ytterbium(II) iodide
Ytterbium(II) iodide is an iodide of ytterbium, with the chemical formula of YbI2. It is a yellow solid.
Preparation
Ytterbium(II) iodide can be prepared by heating ytterbium(III) iodide:
:\mathrm
It can also be prepared by reacting metalli ...
(YbI2) may be used, like samarium(II) iodide
Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and solutions are green as well. It is a strong one-electron reducing a ...
, as a reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth me ...
for coupling reactions A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
. Ytterbium(III) fluoride (YbF3) is used as an inert and non-toxic tooth filling as it continuously releases fluoride ions, which are good for dental health, and is also a good X-ray contrast agent.Enghag, Per (2004). ''Encyclopedia of the elements: technical data, history, processing, applications.'' John Wiley & Sons, p. 448
Oxides
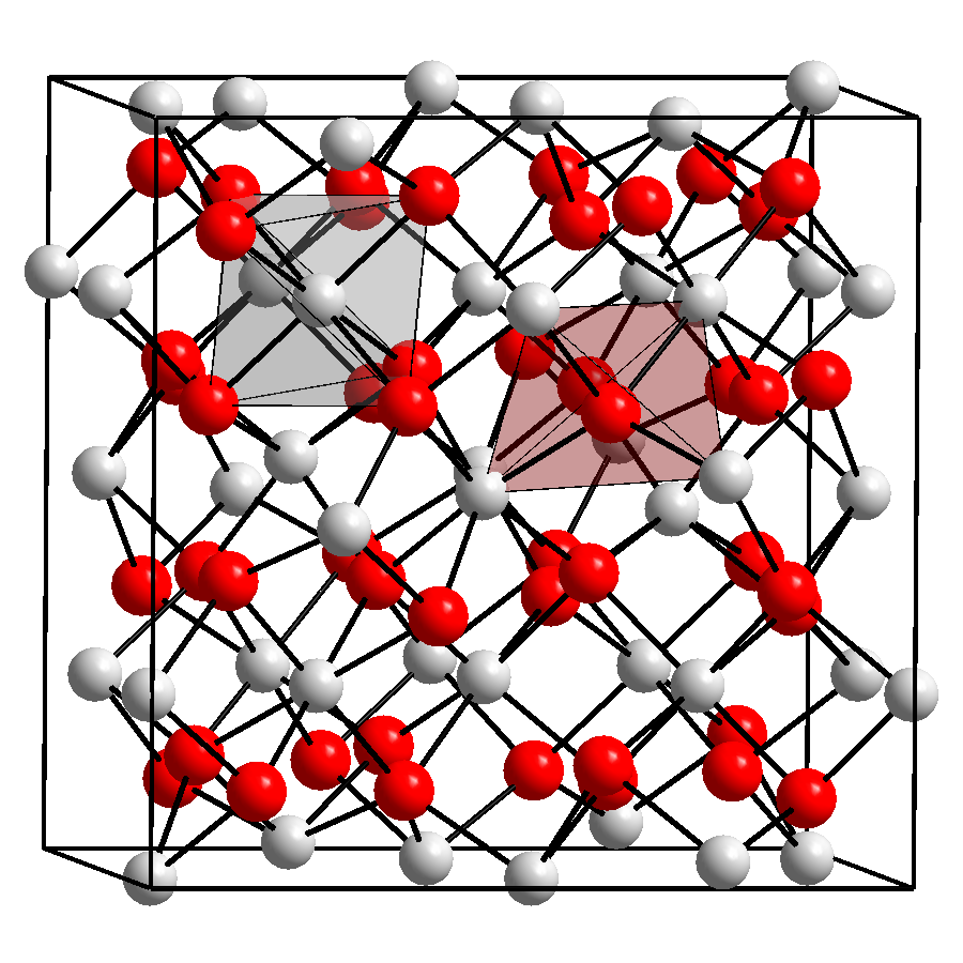
Ytterbium reacts with oxygen to formytterbium(III) oxide
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quart ...
(Yb2O3), which crystallizes in the "rare-earth C-type sesquioxide" structure which is related to the fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs sca ...
structure with one quarter of the anions removed, leading to ytterbium atoms in two different six coordinate (non-octahedral) environments. Ytterbium(III) oxide can be reduced to ytterbium(II) oxide (YbO) with elemental ytterbium, which crystallizes in the same structure as sodium chloride.
Borides
Ytterbium dodecaboride (YbB12) is a crystalline material that has been studied to understand various electronic and structural properties of many chemically related substances. It is aKondo insulator
In solid-state physics, Kondo insulators (also referred as Kondo semiconductors and heavy fermion semiconductors) are understood as materials with strongly correlated electrons, that open up a narrow band gap (in the order of 10 meV) at low t ...
. It is a quantum material; under normal conditions, the interior of the bulk crystal is an insulator whereas the surface is highly conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. Electric current is gene ...
. Among the rare earth elements, ytterbium is one of the few that can form a stable dodecaboride, a property attributed to its comparatively small atomic radius.
History
 Ytterbium was discovered by the Swiss chemist
Ytterbium was discovered by the Swiss chemist Jean Charles Galissard de Marignac
Jean Charles Galissard de Marignac (24 April 1817 – 15 April 1894) was a Swiss chemist whose work with atomic weights suggested the possibility of isotopes and the packing fraction of nuclei. His study of the rare earth elements led to h ...
in the year 1878. While examining samples of gadolinite, Marignac found a new component in the earth then known as erbia
Erbium(III) oxide is the inorganic compound with the formula . It is a pink paramagnetic solid. It finds uses in various optical materials.
Structure
Erbium(III) oxide has a cubic structure resembling the bixbyite motif. The Er3+ centers are o ...
, and he named it ytterbia, for Ytterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is per ...
, the Swedish
Swedish or ' may refer to:
Anything from or related to Sweden, a country in Northern Europe. Or, specifically:
* Swedish language, a North Germanic language spoken primarily in Sweden and Finland
** Swedish alphabet, the official alphabet used by ...
village near where he found the new component of erbium. Marignac suspected that ytterbia was a compound of a new element that he called "ytterbium".
In 1907, the French chemist Georges Urbain Georges Urbain (12 April 1872 – 5 November 1938) was a French chemist, a professor of the Sorbonne, a member of the Institut de France, and director of the Institute of Chemistry in Paris. Much of his work focused on the rare earths, isolating a ...
separated Marignac's ytterbia into two components: neoytterbia and lutecia. Neoytterbia later became known as the element ytterbium, and lutecia became known as the element lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
. The Austrian chemist Carl Auer von Welsbach
Carl Auer von Welsbach (1 September 1858 – 4 August 1929), who received the Austrian noble title of Freiherr Auer von Welsbach in 1901, was an Austrian scientist and inventor, who separated didymium into the elements neodymium and praseo ...
independently isolated these elements from ytterbia at about the same time, but he called them aldebaranium and cassiopeium; the American chemist Charles James Charles James may refer to:
* Charles James (British Army officer) (1757/8–1821), English army officer and writer
* Charles James (attorney) (born 1954), former U.S. assistant attorney general
* Charles James (American football) (born 1990), Amer ...
also independently isolated these elements at about the same time. Urbain and Welsbach accused each other of publishing results based on the other party. The Commission on Atomic Mass, consisting of Frank Wigglesworth Clarke, Wilhelm Ostwald
Friedrich Wilhelm Ostwald (; 4 April 1932) was a Baltic German chemist and philosopher. Ostwald is credited with being one of the founders of the field of physical chemistry, with Jacobus Henricus van 't Hoff, Walther Nernst, and Svante Arrhen ...
, and Georges Urbain, which was then responsible for the attribution of new element names, settled the dispute in 1909 by granting priority to Urbain and adopting his names as official ones, based on the fact that the separation of lutetium from Marignac's ytterbium was first described by Urbain. After Urbain's names were recognized, neoytterbium was reverted to ytterbium.
The chemical and physical properties of ytterbium could not be determined with any precision until 1953, when the first nearly pure ytterbium metal was produced by using ion-exchange processes. The price of ytterbium was relatively stable between 1953 and 1998 at about US$1,000/kg.
Applications
Source of gamma rays
The 169Ybisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
(with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 32 days), which is created along with the short-lived 175Yb isotope (half-life 4.2 days) by neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emit ...
during the irradiation
Irradiation is the process by which an object is exposed to radiation. The exposure can originate from various sources, including natural sources. Most frequently the term refers to ionizing radiation, and to a level of radiation that will serve ...
of ytterbium in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s, has been used as a radiation source in portable X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
machines. Like X-rays, the gamma rays
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
emitted by the source pass through soft tissues of the body, but are blocked by bones and other dense materials. Thus, small 169Yb samples (which emit gamma rays) act like tiny X-ray machines useful for radiography
Radiography is an imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical radiography ("diagnostic" and "therapeu ...
of small objects. Experiments show that radiographs taken with a 169Yb source are roughly equivalent to those taken with X-rays having energies between 250 and 350 keV. 169Yb is also used in nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
.
High-stability atomic clocks
Ytterbium clocks hold the record for stability with ticks stable to within less than two parts in 1 quintillion (). The clocks developed at the National Institute of Standards and Technology (NIST) rely on about 10,000 rare-earth atoms cooled to 10 microkelvin (10 millionths of a degree above absolute zero) and trapped in anoptical lattice
An optical lattice is formed by the interference of counter-propagating laser beams, creating a spatially periodic polarization pattern. The resulting periodic potential may trap neutral atoms via the Stark shift. Atoms are cooled and congrega ...
—a series of pancake-shaped wells made of laser light. Another laser that "ticks" 518 trillion times per second provokes a transition between two energy levels in the atoms. The large number of atoms is key to the clocks' high stability.
Visible light waves oscillate faster than microwaves, and therefore optical clocks can be more precise than caesium atomic clocks
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions betwee ...
. The Physikalisch-Technische Bundesanstalt is working on several such optical clocks. The model with one single ytterbium ion caught in an ion trap
An ion trap is a combination of electric and/or magnetic fields used to capture charged particles — known as ions — often in a system isolated from an external environment. Atomic and molecular ion traps have a number of applications in phy ...
is highly accurate. The optical clock based on it is exact to 17 digits after the decimal point.
A pair of experimental atomic clocks based on ytterbium atoms at the National Institute of Standards and Technology has set a record for stability. NIST physicists reported in the August 22, 2013 issue of Science Express that the ytterbium clocks' ticks are stable to within less than two parts in 1 quintillion
Two naming scales for large numbers have been used in English and other European languages since the early modern era: the long and short scales. Most English variants use the short scale today, but the long scale remains dominant in many non-E ...
(1 followed by 18 zeros), roughly 10 times better than the previous best published results for other atomic clocks. The clocks would be accurate within a second for a period comparable to the age of the universe.
Doping of stainless steel
Ytterbium can also be used as adopant
A dopant, also called a doping agent, is a trace of impurity element that is introduced into a chemical material to alter its original electrical or optical properties. The amount of dopant necessary to cause changes is typically very low. When ...
to help improve the grain refinement, strength, and other mechanical properties of stainless steel. Some ytterbium alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s have rarely been used in dentistry.
Ytterbium as dopant of active media
The Yb3+ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
is used as a doping material in active laser media, specifically in solid state laser
A solid-state laser is a laser that uses a gain medium that is a solid, rather than a liquid as in dye lasers or a gas as in gas lasers. Semiconductor-based lasers are also in the solid state, but are generally considered as a separate class fr ...
s and double clad fiber lasers. Ytterbium lasers are highly efficient, have long lifetimes and can generate short pulses; ytterbium can also easily be incorporated into the material used to make the laser. Ytterbium lasers commonly radiate in the 1.06–1.12 µm band being optically pumped at wavelength 900 nm–1 µm, dependently on the host and application. The small quantum defect The term quantum defect refers to two concepts: energy loss in lasers and energy levels in alkali elements. Both deal with quantum systems where matter interacts with light.
In laser science
In laser science, the term "quantum defect" refers to t ...
makes ytterbium a prospective dopant for efficient lasers and power scaling Power scaling of a laser is increasing its output power without changing the geometry, shape, or principle of operation. Power scalability is considered an important advantage in a laser design. this means it can increase power without changing outs ...
.
The kinetic of excitations in ytterbium-doped materials is simple and can be described within the concept of effective cross-sections; for most ytterbium-doped laser materials (as for many other optically pumped gain media), the McCumber relation holds,
although the application to the ytterbium-doped composite materials
A composite material (also called a composition material or shortened to composite, which is the common name) is a material which is produced from two or more constituent materials. These constituent materials have notably dissimilar chemical or ...
was under discussion.
Usually, low concentrations of ytterbium are used. At high concentrations, the ytterbium-doped materials show photodarkening
(glass fibers) or even a switch to broadband emission (crystals and ceramics) instead of efficient laser action. This effect may be related with not only overheating, but also with conditions of charge compensation at high concentrations of ytterbium ions.
Much progress has been made in the power scaling lasers and amplifiers produced with ytterbium (Yb) doped optical fibers. Power levels have increased from the 1 kW regimes due to the advancements in components as well as the Yb-doped fibers. Fabrication of Low NA, Large Mode Area fibers enable achievement of near perfect beam qualities (M2<1.1) at power levels of 1.5 kW to greater than 2 kW at ~1064 nm in a broadband configuration. Ytterbium-doped LMA fibers also have the advantages of a larger mode field diameter, which negates the impacts of nonlinear effects such as stimulated Brillouin scattering Brillouin scattering (also known as Brillouin light scattering or BLS), named after Léon Brillouin, refers to the interaction of light with the material waves in a medium (e.g. electrostriction and magnetostriction). It is mediated by the refractiv ...
and stimulated Raman scattering
Raman scattering or the Raman effect () is the inelastic scattering of photons by matter, meaning that there is both an exchange of energy and a change in the light's direction. Typically this effect involves vibrational energy being gained by a ...
, which limit the achievement of higher power levels, and provide a distinct advantage over single mode ytterbium-doped fibers.
In order to achieve even higher power levels in ytterbium-based fiber systems. all factors of the fiber must be considered. These can be achieved only via optimization of all the ytterbium fiber parameters, ranging from the core background losses to the geometrical properties, in order to reduce the splice losses within the cavity. Power scaling also requires optimization of matching passive fibers within the optical cavity. The optimization of the ytterbium-doped glass itself through host glass modification of various dopants also plays a large part in reducing the background loss of the glass, improvements in slope efficiency of the fiber, and improved photodarkening performance, all of which contribute to increased power levels in 1 µm systems.
Ion Qubits for Quantum Computing
The charged ion 171Yb+ is used in trapped-ion qubits in quantum computing. Entanglinggates
Gates is the plural of gate, a point of entry to a space which is enclosed by walls. It may also refer to:
People
* Gates (surname), various people with the last name
* Gates Brown (1939-2013), American Major League Baseball player
* Gates McFadde ...
, such as the Mølmer–Sørensen gate, have been achieved by addressing the ions with mode-locked pulse lasers.
Others
Ytterbium metal increases its electrical resistivity when subjected to high stresses. This property is used in stress gauges to monitor ground deformations from earthquakes and explosions. Currently, ytterbium is being investigated as a possible replacement formagnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
in high density pyrotechnic payloads for kinematic infrared decoy flares. As ytterbium(III) oxide
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quart ...
has a significantly higher emissivity
The emissivity of the surface of a material is its effectiveness in emitting energy as thermal radiation. Thermal radiation is electromagnetic radiation that most commonly includes both visible radiation (light) and infrared radiation, which is n ...
in the infrared range than magnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ...
, a higher radiant intensity is obtained with ytterbium-based payloads in comparison to those commonly based on magnesium/Teflon/Viton (MTV).
Precautions
Although ytterbium is fairly stable chemically, it is stored in airtight containers and in an inert atmosphere such as a nitrogen-filled dry box to protect it from air and moisture. All compounds of ytterbium are treated as highlytoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
, although studies appear to indicate that the danger is minimal. However, ytterbium compounds cause irritation to human skin and eyes, and some might be teratogenic
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology. The related t ...
. Metallic ytterbium dust can spontaneously combust, and the resulting fumes are hazardous. Ytterbium fires cannot be extinguished using water, and only dry chemical class D fire extinguisher
A fire extinguisher is a handheld active fire protection device usually filled with a dry or wet chemical used to extinguish or control small fires, often in emergencies. It is not intended for use on an out-of-control fire, such as one which ha ...
s can extinguish the fires.
References
Further reading
*''Guide to the Elements – Revised Edition'', Albert Stwertka, (Oxford University Press; 1998)External links
It's Elemental – Ytterbium
*
Encyclopedia of Geochemistry - Ytterbium
{{Good article Chemical elements Chemical elements with face-centered cubic structure Lanthanides Suspected teratogens