Rutile needles.jpg on:
[Wikipedia]
[Google]
[Amazon]
Rutile is an
 Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment particularly as products of
Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment particularly as products of
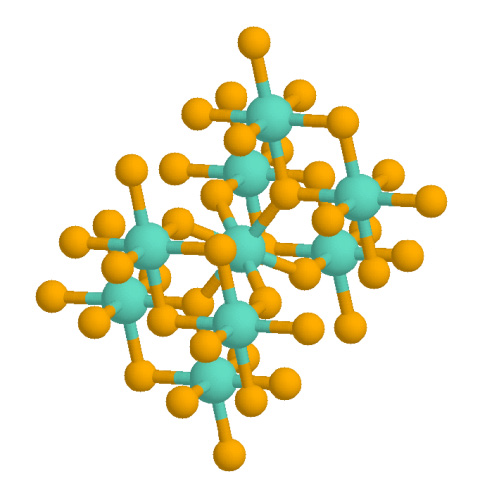 Rutile has a tetragonal unit cell, with unit cell parameters ''a'' = ''b'' = 4.584 Å, and ''c'' = 2.953 Å. The titanium cations have a coordination number of 6, meaning they are surrounded by an octahedron of 6 oxygen atoms. The oxygen anions have a coordination number of 3, resulting in a trigonal planar coordination. Rutile also shows a screw axis when its octahedra are viewed sequentially. When formed under reducing conditions, oxygen vacancies can occur, coupled to Ti3+ centers. Hydrogen can enter these gaps, existing as an individual vacancy occupant (pairing as a hydrogen ion) or creating a hydroxide group with an adjacent oxygen.
Rutile crystals are most commonly observed to exhibit a prismatic or acicular growth habit with preferential orientation along their ''c'' axis, the 01 direction. This growth habit is favored as the facets of rutile exhibit the lowest
Rutile has a tetragonal unit cell, with unit cell parameters ''a'' = ''b'' = 4.584 Å, and ''c'' = 2.953 Å. The titanium cations have a coordination number of 6, meaning they are surrounded by an octahedron of 6 oxygen atoms. The oxygen anions have a coordination number of 3, resulting in a trigonal planar coordination. Rutile also shows a screw axis when its octahedra are viewed sequentially. When formed under reducing conditions, oxygen vacancies can occur, coupled to Ti3+ centers. Hydrogen can enter these gaps, existing as an individual vacancy occupant (pairing as a hydrogen ion) or creating a hydroxide group with an adjacent oxygen.
Rutile crystals are most commonly observed to exhibit a prismatic or acicular growth habit with preferential orientation along their ''c'' axis, the 01 direction. This growth habit is favored as the facets of rutile exhibit the lowest
 In large enough quantities in beach sands, rutile forms an important constituent of
In large enough quantities in beach sands, rutile forms an important constituent of
Magnetism in titanium dioxide polymorphs
J. Applied Physics Research efforts typically utilize small quantities of synthetic rutile rather than mineral-deposit derived materials.
oxide mineral
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. The minerals with complex anion groups such as the si ...
composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wavelength
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' or simply light. A typical human eye will respond to wave ...
s of any known crystal and also exhibits a particularly large birefringence
Birefringence is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are said to be birefringent (or birefractive). The birefring ...
and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization
Polarization or polarisation may refer to:
Mathematics
*Polarization of an Abelian variety, in the mathematics of complex manifolds
*Polarization of an algebraic form, a technique for expressing a homogeneous polynomial in a simpler fashion by ...
optics, for longer visible
Visibility, in meteorology, is a measure of the distance at which an object or light can be seen.
Visibility may also refer to:
* A measure of turbidity in water quality control
* Interferometric visibility, which quantifies interference contrast ...
and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
and tantalum.
Rutile derives its name from the Latin ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner.
Occurrence
Rutile is a common accessory mineral in high-temperature and high-pressure metamorphic rocks and in igneous rocks. Thermodynamically, rutile is the most stable polymorph of TiO2 at all temperatures, exhibiting lower total free energy than metastable phases of anatase or brookite. Consequently, the transformation of the metastable TiO2 polymorphs to rutile is irreversible. As it has the lowestmolecular volume
The Van der Waals surface of a molecule is an abstract representation or model of that molecule, illustrating where, in very rough terms, a surface might reside for the molecule based on the hard cutoffs of Van der Waals radii for individual at ...
of the three main polymorphs, it is generally the primary titanium-bearing phase in most high-pressure metamorphic rocks, chiefly eclogites.
 Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment particularly as products of
Within the igneous environment, rutile is a common accessory mineral in plutonic igneous rocks, though it is also found occasionally in extrusive igneous rocks, particularly those such as kimberlites and lamproites that have deep mantle sources. Anatase and brookite are found in the igneous environment particularly as products of autogenic alteration Autogenesis may refer to:
* Abiogenesis, the origination of life from non-living things, as believed by Aristotle and in modern evolutionary theory
* Orthogenesis, a discredited evolutionary idea that hypothesised a directed teleological form of evo ...
during the cooling of plutonic rocks; anatase is also found in placer deposits sourced from primary rutile.
The occurrence of large specimen crystals is most common in pegmatite
A pegmatite is an igneous rock showing a very coarse texture, with large interlocking crystals usually greater in size than and sometimes greater than . Most pegmatites are composed of quartz, feldspar, and mica, having a similar silicic com ...
s, skarns, and granite greisens. Rutile is found as an accessory mineral in some altered igneous rocks, and in certain gneisses and schists. In groups of acicular crystals it is frequently seen penetrating quartz as in the from Graubünden, Switzerland
). Swiss law does not designate a ''capital'' as such, but the federal parliament and government are installed in Bern, while other federal institutions, such as the federal courts, are in other cities (Bellinzona, Lausanne, Luzern, Neuchâtel ...
. In 2005 the Republic of Sierra Leone in West Africa had a production capacity of 23% of the world's annual rutile supply, which rose to approximately 30% in 2008.
Crystal structure
 Rutile has a tetragonal unit cell, with unit cell parameters ''a'' = ''b'' = 4.584 Å, and ''c'' = 2.953 Å. The titanium cations have a coordination number of 6, meaning they are surrounded by an octahedron of 6 oxygen atoms. The oxygen anions have a coordination number of 3, resulting in a trigonal planar coordination. Rutile also shows a screw axis when its octahedra are viewed sequentially. When formed under reducing conditions, oxygen vacancies can occur, coupled to Ti3+ centers. Hydrogen can enter these gaps, existing as an individual vacancy occupant (pairing as a hydrogen ion) or creating a hydroxide group with an adjacent oxygen.
Rutile crystals are most commonly observed to exhibit a prismatic or acicular growth habit with preferential orientation along their ''c'' axis, the 01 direction. This growth habit is favored as the facets of rutile exhibit the lowest
Rutile has a tetragonal unit cell, with unit cell parameters ''a'' = ''b'' = 4.584 Å, and ''c'' = 2.953 Å. The titanium cations have a coordination number of 6, meaning they are surrounded by an octahedron of 6 oxygen atoms. The oxygen anions have a coordination number of 3, resulting in a trigonal planar coordination. Rutile also shows a screw axis when its octahedra are viewed sequentially. When formed under reducing conditions, oxygen vacancies can occur, coupled to Ti3+ centers. Hydrogen can enter these gaps, existing as an individual vacancy occupant (pairing as a hydrogen ion) or creating a hydroxide group with an adjacent oxygen.
Rutile crystals are most commonly observed to exhibit a prismatic or acicular growth habit with preferential orientation along their ''c'' axis, the 01 direction. This growth habit is favored as the facets of rutile exhibit the lowest surface free energy
In surface science, surface free energy (also interfacial free energy or surface energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energ ...
and are therefore thermodynamically most stable. The ''c''-axis oriented growth of rutile appears clearly in nanorods, nanowires and abnormal grain growth phenomena of this phase.
Application
 In large enough quantities in beach sands, rutile forms an important constituent of
In large enough quantities in beach sands, rutile forms an important constituent of heavy mineral In geology, a heavy mineral is a mineral with a density that is greater than 2.9 g/cm3, most commonly referring to dense components of siliciclastic sediments. A heavy mineral suite is the relative percentages of heavy minerals in a stone. Heavy min ...
s and ore deposits. Miners extract and separate the valuable minerals – e.g., rutile, zircon, and ilmenite. The main uses for rutile are the manufacture of refractory ceramic
In materials science, a refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, ...
, as a pigment, and for the production of titanium metal.
Finely powdered rutile is a brilliant white pigment and is used in paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s, plastics, paper, foods, and other applications that call for a bright white color. Titanium dioxide pigment is the single greatest use of titanium worldwide. Nanoscale particles of rutile are transparent to visible light but are highly effective in the absorption
Absorption may refer to:
Chemistry and biology
* Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which ...
of ultraviolet radiation (sunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn and ...
). The UV absorption of nano-sized rutile particles is blue-shifted compared to bulk rutile, so that higher-energy UV light is absorbed by the nanoparticles. Hence, they are used in sunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn and ...
s to protect against UV-induced skin damage.
Small rutile needles present in gems
Gems, or gemstones, are polished, cut stones or minerals.
Gems or GEMS may also refer to:
Arts, entertainment and media
*Gems (Aerosmith album), ''Gems'' (Aerosmith album), 1988
*Gems (Patti LaBelle album), ''Gems'' (Patti LaBelle album), 1994
*G ...
are responsible for an optical phenomenon known as asterism. Asteriated gems are known as "star" gems. Star sapphires, star rubies, and other star gems are highly sought after and are generally more valuable than their normal counterparts.
Rutile is widely used as a welding electrode covering. It is also used as a part of the ZTR index
The ZTR index is a method of determining how weathered,Prothero, D. R. and Schwab, F., 1996, Sedimentary Geology, pg. 460, both chemically and mechanically, a sediment (or a corresponding sedimentary rock) is. The letters in ZTR stand for 3 com ...
, which classifies highly weathered sediments.
Semiconductor
Rutile, as a large band-gap semiconductor, has in recent decades been the subject of significant research towards applications as a functional oxide for applications in photocatalysis and dilute magnetism.J. Applied Physics Research efforts typically utilize small quantities of synthetic rutile rather than mineral-deposit derived materials.
Synthetic rutile
Synthetic rutile was first produced in 1948 and is sold under a variety of names. It can be produced from the titanium ore ilmenite through theBecher process The Becher process is an industrial process used to produce rutile, a form of titanium dioxide, from the ore ilmenite. It is competitive with the chloride process and the sulfate process, which achieve similar net conversions.
With the idealized fo ...
. Very pure synthetic rutile is transparent and almost colorless, being slightly yellow, in large pieces. Synthetic rutile can be made in a variety of colors by doping. The high refractive index gives an adamantine luster and strong refraction that leads to a diamond-like appearance. The near-colorless diamond substitute is sold as "Titania", which is the old-fashioned chemical name for this oxide. However, rutile is seldom used in jewellery because it is not very hard
Hard may refer to:
* Hardness, resistance of physical materials to deformation or fracture
* Hard water, water with high mineral content
Arts and entertainment
* ''Hard'' (TV series), a French TV series
* Hard (band), a Hungarian hard rock super ...
(scratch-resistant), measuring only about 6 on the Mohs hardness scale.
As the result of growing research interest in the photocatalytic
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the abi ...
activity of titanium dioxide, in both anatase and rutile phases (as well as biphasic mixtures of the two phases), rutile TiO2 in powder and thin film form is frequently fabricated in laboratory conditions through solution based routes using inorganic precursors (typically TiCl4) or organometallic precursors (typically alkoxides such as titanium isopropoxide, also known as TTIP). Depending on synthesis conditions, the first phase to crystallize may be the metastable anatase phase, which can then be converted to the equilibrium rutile phase through thermal treatment. The physical properties of rutile are often modified using dopants to impart improved photocatalytic activity through improved photo-generated charge carrier separation, altered electronic band structures and improved surface reactivity.
See also
* List of mineralsReferences
External links
* {{Authority control Titanium minerals Oxide minerals Rutile group Tetragonal minerals Minerals in space group 136