Phenol chemical structure.png on:
[Wikipedia]
[Google]
[Amazon]
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular
C6H5OH <=> C6H5O- + H+
 Phenol is more acidic than aliphatic alcohols. The differing pKa is attributed to resonance stabilization of the phenoxide anion. In this way, the negative charge on oxygen is delocalized on to the ortho and para carbon atoms through the pi system. An alternative explanation involves the sigma framework, postulating that the dominant effect is the
Phenol is more acidic than aliphatic alcohols. The differing pKa is attributed to resonance stabilization of the phenoxide anion. In this way, the negative charge on oxygen is delocalized on to the ortho and para carbon atoms through the pi system. An alternative explanation involves the sigma framework, postulating that the dominant effect is the
 Phenol exhibits keto-enol tautomerism with its unstable keto tautomer cyclohexadienone, but only a tiny fraction of phenol exists as the keto form. The equilibrium constant for enolisation is approximately 10−13, which means only one in every ten trillion molecules is in the keto form at any moment. The small amount of stabilisation gained by exchanging a C=C bond for a C=O bond is more than offset by the large destabilisation resulting from the loss of aromaticity. Phenol therefore exists essentially entirely in the enol form. 4, 4' Substituted cyclohexadienone can undergo a
Phenol exhibits keto-enol tautomerism with its unstable keto tautomer cyclohexadienone, but only a tiny fraction of phenol exists as the keto form. The equilibrium constant for enolisation is approximately 10−13, which means only one in every ten trillion molecules is in the keto form at any moment. The small amount of stabilisation gained by exchanging a C=C bond for a C=O bond is more than offset by the large destabilisation resulting from the loss of aromaticity. Phenol therefore exists essentially entirely in the enol form. 4, 4' Substituted cyclohexadienone can undergo a
 Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via
Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via C6H5OH + NaOH -> C6H5ONa + H2O
When a mixture of phenol and C6H5COCl + HOC6H5 -> C6H5CO2C6H5 + HCl
Phenol is reduced to benzene when it is distilled with zinc dust or when its vapour is passed over granules of zinc at 400 °C:
:C6H5OH + Zn -> C6H6 + ZnO
When phenol is treated with diazomethane in the presence of C6H5OH + CH2N2 -> C6H5OCH3 + N2
When phenol reacts with iron(III) chloride solution, an intense violet-purple solution is formed.
 Accounting for 95% of production (2003) is the cumene process, also called Hock process. It involves the partial oxidation of cumene (isopropylbenzene) via the
Accounting for 95% of production (2003) is the cumene process, also called Hock process. It involves the partial oxidation of cumene (isopropylbenzene) via the
C6H6 + O -> C6H5OH
Nitrous oxide is a potentially "green" oxidant that is a more potent oxidant than O2. Routes for the generation of nitrous oxide however remain uncompetitive.
An electrosynthesis employing alternating current gives phenol from benzene.
The oxidation of toluene, as developed by C6H5CH3 + 2 O2 -> C6H5OH + CO2 + H2O
The reaction is proposed to proceed via formation of benzyoylsalicylate.
C6H5SO3H + 2 NaOH -> C6H5OH + Na2SO3 + H2O
C6H5Cl + NaOH -> C6H5OH + NaCl
:C6H5Cl + H2O -> C6H5OH + HCl
These methods suffer from the cost of the chlorobenzene and the need to dispose of the chloride by product.
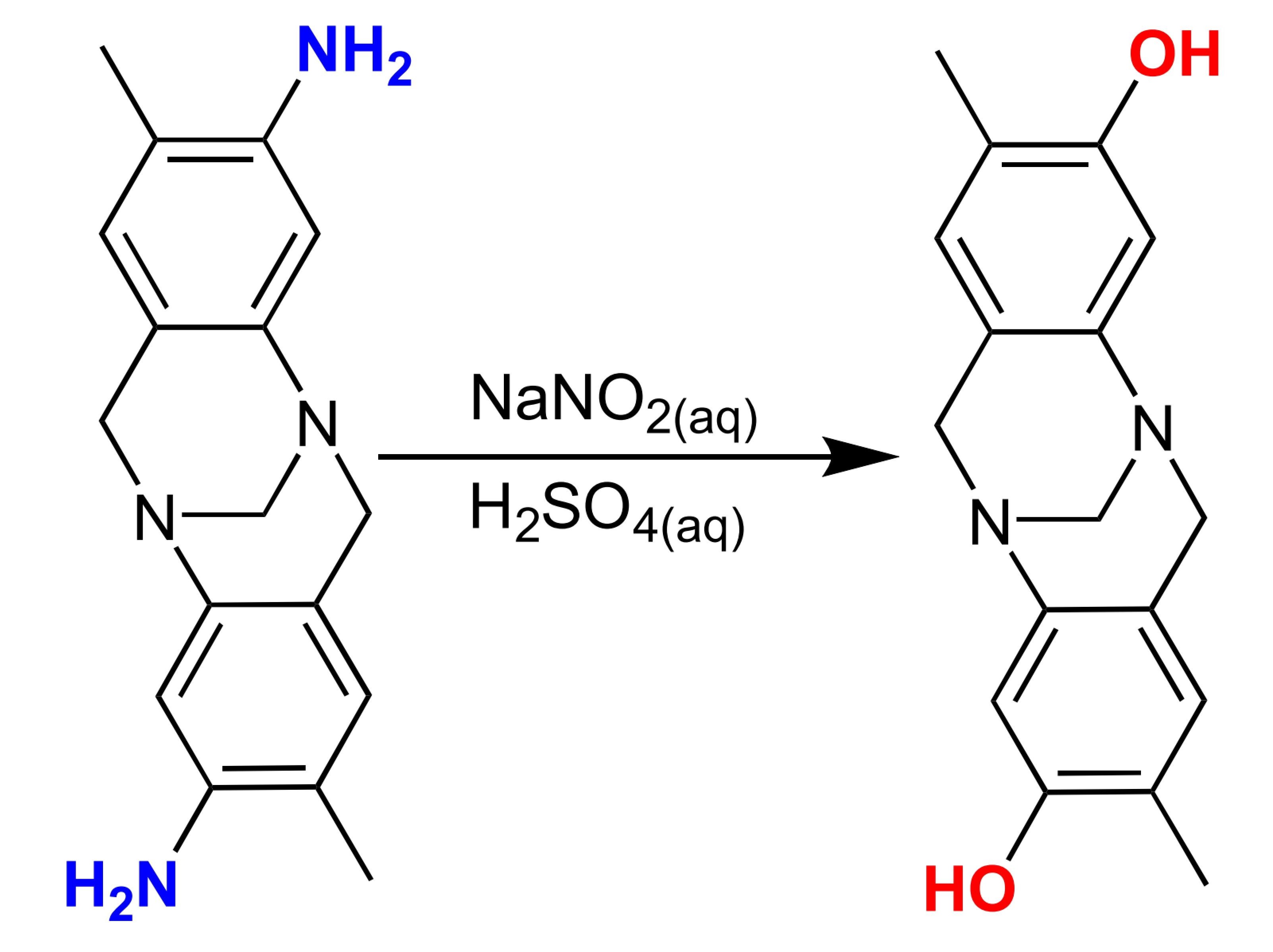
C6H5NH2 + HCl/NaNO2 -> C6H5OH + N2 + H2O + NaCl
:
by Peter Tyson. NOVA It was originally used by the Nazis in 1939 as part of the Aktion T4 euthanasia program.''The Nazi Doctors''
, Chapter 14, Killing with Syringes: Phenol Injections. By Dr. Robert Jay Lifton The Germans learned that extermination of smaller groups was more economical by injection of each victim with phenol. Phenol injections were given to thousands of people. Maximilian Kolbe was also killed with a phenol injection after surviving two weeks of dehydration and starvation in
International Chemical Safety Card 0070National Pollutant Inventory: Phenol Fact SheetCDC - Phenol - NIOSH Workplace Safety and Health Topic
{{Authority control Antiseptics Commodity chemicals Hazardous air pollutants Oxoacids Phenyl compounds 1834 in science
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
() bonded to a hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
(). Mildly acidic, it requires careful handling because it can cause chemical burns.
Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity
In economics, a commodity is an economic good, usually a resource, that has full or substantial fungibility: that is, the market treats instances of the good as equivalent or nearly so with no regard to who produced them.
The price of a comm ...
as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics
Plastics are a wide range of synthetic polymers, synthetic or semi-synthetic materials that use polymers as a main ingredient. Their Plasticity (physics), plasticity makes it possible for plastics to be Injection moulding, moulded, Extrusion, e ...
and related materials. Phenol and its chemical derivatives
The derivative of a function is the rate of change of the function's output relative to its input value.
Derivative may also refer to:
In mathematics and economics
* Brzozowski derivative in the theory of formal languages
* Formal derivative, an ...
are essential for production of polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
s, epoxies
The Epoxies were an American New wave music, new wave band from Portland, Oregon, formed in 2000. Heavily influenced by new wave, the band jokingly described themselves as robot garage rock. Members included FM Static on synthesizers, guitarist ...
, Bakelite
Polyoxybenzylmethylenglycolanhydride, better known as Bakelite ( ), is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed ...
, nylon, detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more ...
s, herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s such as phenoxy herbicides, and numerous pharmaceutical drugs.
Properties
Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol to water mass ratios of ~2.6 and higher are possible. The sodium salt of phenol,sodium phenoxide
Sodium phenoxide (sodium phenolate) is an organic compound with the formula NaOC6H5. It is a white crystalline solid. Its anion, phenoxide, also known as phenolate, is the conjugate base of phenol. It is used as a precursor to many other organic ...
, is far more water-soluble.
Acidity
Phenol is a weak acid. In aqueous solution in the pH range ca. 8 - 12 it is in equilibrium with the phenolate anion (also called phenoxide): :induction
Induction, Inducible or Inductive may refer to:
Biology and medicine
* Labor induction (birth/pregnancy)
* Induction chemotherapy, in medicine
* Induced stem cells, stem cells derived from somatic, reproductive, pluripotent or other cell t ...
from the more electronegative sp2 hybridised carbons; the comparatively more powerful inductive withdrawal of electron density that is provided by the sp2 system compared to an sp3 system allows for great stabilization of the oxyanion. In support of the second explanation, the p''K''a of the enol of acetone in water is 10.9, making it only slightly less acidic than phenol (p''K''a 10.0). Thus, the greater number of resonance structures available to phenoxide compared to acetone enolate seems to contribute very little to its stabilization. However, the situation changes when solvation effects are excluded. A recent ''in silico'' comparison of the gas phase acidities of the vinylogues of phenol and cyclohexanol in conformations that allow for or exclude resonance stabilization leads to the inference that about of the increased acidity of phenol is attributable to inductive effects, with resonance accounting for the remaining difference.
Hydrogen bonding
Incarbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVAC ...
and alkane solvents phenol hydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
with a wide range of Lewis bases such as pyridine, diethyl ether, and diethyl sulfide
Diethyl sulfide (British English: diethyl sulphide) is an organosulfur compound with the chemical formula . It is a colorless, malodorous liquid. Although a common thioether, it has few applications.
Preparation
Diethyl sulfide is a by-product ...
. The enthalpies of adduct formation and the IR frequency shifts accompanying adduct formation have been studied. Phenol is classified as a hard acid which is compatible with the ''C''/''E'' ratio of the ''ECW'' model with ''E''A = 2.27 and ''C''A = 1.07. The relative acceptor strength of phenol toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots.
Phenoxide anion
The phenoxide anion is a strongnucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
with a nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
comparable to the one of carbanions or tertiary amines. It can react at both its oxygen or carbon sites as an ambident nucleophile (see HSAB theory). Generally, oxygen attack of phenoxide anions is kinetically favored, while carbon-attack is thermodynamically preferred (see Thermodynamic versus kinetic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the conversion (che ...
). Mixed oxygen/carbon attack and by this a loss of selectivity is usually observed if the reaction rate reaches diffusion control.
Tautomerism
dienone–phenol rearrangement
The dienone–phenol rearrangement is a reaction in organic chemistry first reported in 1921 by Karl von Auwers and Karl Ziegler. A common example of dienone–phenol rearrangement is 4,4-disubstituted converting into a stable 3,4-disubstituted ph ...
in acid conditions and form stable 3,4‐disubstituted phenol.
Phenoxides are enolates stabilised by aromaticity. Under normal circumstances, phenoxide is more reactive at the oxygen position, but the oxygen position is a "hard" nucleophile whereas the alpha-carbon positions tend to be "soft".
Reactions
 Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via
Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, ...
, acylation, sulfonation, and related processes. Phenol's ring is so strongly activated that bromination and chlorination lead readily to polysubstitution. Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, additional nitro groups are introduced, e.g. to give 2,4,6-trinitrophenol
Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from el, πικρός (''pikros''), meaning "bitter", due to its bitter taste. It is one of the most acidic ...
.
Aqueous solutions of phenol are weakly acidic and turn blue litmus slightly to red. Phenol is neutralized by sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
forming sodium phenate or phenolate, but being weaker than carbonic acid, it cannot be neutralized by sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
or sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
to liberate carbon dioxide.
:benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It i ...
are shaken in presence of dilute sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
solution, phenyl benzoate
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic compound, cyclic group of atoms with the formula carbon, C6hydrogen, H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as ...
is formed. This is an example of the Schotten–Baumann reaction
The Schotten–Baumann reaction is a method to synthesize amides from amines and acid chlorides:
Schotten–Baumann reaction also refers to the conversion of acid chloride to esters. The reaction was first described in 1883 by German chemists ...
:
:boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
(), anisole is obtained as the main product and nitrogen gas as a byproduct.
:Production
Because of phenol's commercial importance, many methods have been developed for its production, but the cumene process is the dominant technology.Cumene process
 Accounting for 95% of production (2003) is the cumene process, also called Hock process. It involves the partial oxidation of cumene (isopropylbenzene) via the
Accounting for 95% of production (2003) is the cumene process, also called Hock process. It involves the partial oxidation of cumene (isopropylbenzene) via the Hock rearrangement
Hock may refer to:
Common meanings:
* Hock (wine), a type of wine
* Hock (anatomy), part of an animal's leg
* To leave an item with a pawnbroker
People:
* Hock (surname)
* Richard "Hock" Walsh (1948-1999), Canadian blues singer
Other uses:
* A ...
: Compared to most other processes, the cumene process uses relatively mild conditions and relatively inexpensive raw materials. For the process to be economical, both phenol and the acetone by-product must be in demand. In 2010, worldwide demand for acetone was approximately 6.7 million tonnes, 83 percent of which was satisfied with acetone produced by the cumene process.
A route analogous to the cumene process begins with cyclohexylbenzene
Cyclohexylbenzene is the organic compound with the structural formula C6H5-C6H11. It is a derivative of benzene with a cyclohexyl substituent (C6H11). It is a colorless liquid.
Formation
Cyclohexylbenzene is produced by the acid-catalyzed alkyla ...
. It is oxidized to a hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have t ...
, akin to the production of cumene hydroperoxide. Via the Hock rearrangement, cyclohexylbenzene hydroperoxide cleaves to give phenol and cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexan ...
. Cyclohexanone is an important precursor to some nylons.
Oxidation of benzene and toluene
The direct oxidation of benzene () to phenol is theoretically possible and of great interest, but it has not been commercialized: :Dow Chemical
The Dow Chemical Company, officially Dow Inc., is an American multinational chemical corporation headquartered in Midland, Michigan, United States. The company is among the three largest chemical producers in the world.
Dow manufactures plastics ...
, involves copper-catalyzed reaction of molten sodium benzoate with air:
:Older methods
Early methods relied on extraction of phenol from coal derivatives or the hydrolysis of benzene derivatives.Hydrolysis of benzenesulfonic acid
An early commercial route, developed byBayer
Bayer AG (, commonly pronounced ; ) is a German multinational corporation, multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of busi ...
and Monsanto in the early 1900s, begins with the reaction of a strong base with benzenesulfonic acid. The conversion is represented by this idealized equation:Wittcoff, H.A., Reuben, B.G. Industrial Organic Chemicals in Perspective. Part One: Raw Materials and Manufacture. Wiley-Interscience, New York. 1980.
:Hydrolysis of chlorobenzene
Chlorobenzene can be hydrolyzed to phenol using base ( Dow process) or steam (Raschig–Hooker process
The Raschig–Hooker process is a chemical process for the production of chlorobenzene and phenol.
The Raschig–Hooker process was patented by Friedrich Raschig, a German chemist and politician also known for the Raschig process, the Olin Rasc ...
):
:Coal pyrolysis
Phenol is also a recoverable byproduct of coal pyrolysis.Franck, H.-G., Stadelhofer, J.W. Industrial Aromatic Chemistry. Springer-Verlag, New York. 1988. pp. 148-155. In theLummus Process Lummus may refer to:
* J. E. Lummus and J. N. Lummus, bankers who moved to Miami in 1895
*Lummus Company, manufacturer of cotton gins, Savannah Georgia
* Lummus Global, a company of Chicago Bridge & Iron Company
* Lummus Island, one of the three is ...
, the oxidation of toluene to benzoic acid is conducted separately.
Miscellaneous methods

Phenyldiazonium
Benzenediazonium tetrafluoroborate is an organic compound with the formula 6H5N2F4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the ary ...
salts hydrolyze to phenol. The method is of no commercial interest since the precursor is expensive.
:Salicylic acid
Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substance ...
decarboxylates to phenol.
Uses
The major uses of phenol, consuming two thirds of its production, involve its conversion to precursors for plastics.Condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
with acetone gives bisphenol-A
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial s ...
, a key precursor to polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
s and epoxide resins. Condensation of phenol, alkylphenols, or diphenols with formaldehyde gives phenolic resins, a famous example of which is Bakelite
Polyoxybenzylmethylenglycolanhydride, better known as Bakelite ( ), is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed ...
. Partial hydrogenation of phenol gives cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexan ...
, a precursor to nylon. Nonionic detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more ...
s are produced by alkylation of phenol to give the alkylphenols, e.g., nonylphenol, which are then subjected to ethoxylation.
Phenol is also a versatile precursor to a large collection of drugs, most notably aspirin but also many herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s and pharmaceutical drugs.
Phenol is a component in liquid–liquid phenol–chloroform extraction technique used in molecular biology for obtaining nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s from tissues or cell culture samples. Depending on the pH of the solution either DNA or RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
can be extracted.
Medical
Phenol is widely used as an antiseptic. Its use was pioneered by Joseph Lister (see section). From the early 1900s to the 1970s it was used in the production ofcarbolic soap
Carbolic soap, sometimes referred to as red soap, is a mildly antiseptic soap containing carbolic acid (phenol) and/or cresylic acid (cresol), both of which are phenols derived from either coal tar or petroleum sources.
History
In 1834, German c ...
. Concentrated phenol liquids are commonly used for permanent treatment of ingrown toe and finger nails, a procedure known as a chemical matrixectomy
Surgical treatments of ingrown toenails include a number of different options. If conservative treatment of a minor ingrown toenail does not succeed or if the ingrown toenail is severe, surgical management by a podiatrist is recommended. The i ...
. The procedure was first described by Otto Boll in 1945. Since that time it has become the chemical of choice for chemical matrixectomies performed by podiatrists.
Concentrated liquid phenol can be used topically as a local anesthetic for otology procedures, such as myringotomy and tympanotomy tube placement, as an alternative to general anesthesia or other local anesthetics. It also has hemostatic and antiseptic qualities that make it ideal for this use.
Phenol spray, usually at 1.4% phenol as an active ingredient, is used medically to treat sore throat. It is the active ingredient in some oral analgesics such as Chloraseptic
Chloraseptic is an American brand of oral analgesic that is produced by Tarrytown, New York-based Prestige Consumer Healthcare, used for the relief of sore throat and mouth pain. Its active ingredient is phenol (just in Sore Throat Spray, not in ...
spray, TCP
TCP may refer to:
Science and technology
* Transformer coupled plasma
* Tool Center Point, see Robot end effector
Computing
* Transmission Control Protocol, a fundamental Internet standard
* Telephony control protocol, a Bluetooth communication s ...
and Carmex
Carmex is a brand of lip balm produced by Carma Laboratories, Inc. It is sold in jars, sticks, and squeezable containers.
History
Carma Laboratories, Inc. began in Wisconsin in the early 1930s when Alfred Woelbing began experimenting with creat ...
.
Niche uses
Phenol is so inexpensive that it attracts many small-scale uses. It is a component of industrial paint strippers used in the aviation industry for the removal of epoxy, polyurethane and other chemically resistant coatings. Phenol derivatives have been used in the preparation of cosmetics includingsunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn and ...
s, hair colorings, and skin lightening
Skin whitening, also known as skin lightening and skin bleaching, is the practice of using chemical substances in an attempt to lighten the skin or provide an even skin color by reducing the melanin concentration in the skin. Several chemicals ha ...
preparations. However, due to safety concerns, phenol is banned from use in cosmetic products in the European Union and Canada.
History
Phenol was discovered in 1834 by Friedlieb Ferdinand Runge, who extracted it (in impure form) from coal tar. Runge called phenol "Karbolsäure" (coal-oil-acid, carbolic acid). Coal tar remained the primary source until the development of the petrochemical industry. The French chemistAuguste Laurent
Auguste Laurent (14 November 1807 – 15 April 1853) was a French chemist who helped in the founding of organic chemistry with his discoveries of anthracene, phthalic acid, and carbolic acid.
He devised a systematic nomenclature for organic chem ...
extracted phenol in its pure form, as a derivative of benzene, in 1841.
In 1836, Auguste Laurent coined the name "phène" for benzene; this is the root of the word "phenol" and " phenyl". In 1843, French chemist Charles Gerhardt coined the name "phénol".
The antiseptic properties of phenol were used by Sir Joseph Lister (1827–1912) in his pioneering technique of antiseptic surgery. Lister decided that the wounds themselves had to be thoroughly cleaned. He then covered the wounds with a piece of rag or lint covered in carbolic acid (phenol). The skin irritation caused by continual exposure to phenol eventually led to the introduction of aseptic (germ-free) techniques in surgery.
Joseph Lister was a student at University College London under Robert Liston, later rising to the rank of Surgeon at Glasgow Royal Infirmary. Lister experimented with cloths covered in carbolic acid after studying the works and experiments of his contemporary, Louis Pasteur in sterilizing various biological media. Lister was inspired to try to find a way to sterilize living wounds, which could not be done with the heat required by Pasteur's experiments. In examining Pasteur's research, Lister began to piece together his theory: that patients were being killed by germs. He theorized that if germs could be killed or prevented, no infection would occur. Lister reasoned that a chemical could be used to destroy the micro-organisms that cause infection.
Meanwhile, in Carlisle, England, officials were experimenting with sewage treatment using carbolic acid to reduce the smell of sewage cesspools. Having heard of these developments, and having himself previously experimented with other chemicals for antiseptic purposes without much success, Lister decided to try carbolic acid as a wound antiseptic. He had his first chance on August 12, 1865, when he received a patient: an eleven-year-old boy with a tibia bone fracture which pierced the skin of his lower leg. Ordinarily, amputation would be the only solution. However, Lister decided to try carbolic acid. After setting the bone and supporting the leg with splints, he soaked clean cotton towels in undiluted carbolic acid and applied them to the wound, covered with a layer of tin foil, leaving them for four days. When he checked the wound, Lister was pleasantly surprised to find no signs of infection, just redness near the edges of the wound from mild burning by the carbolic acid. Reapplying fresh bandages with diluted carbolic acid, the boy was able to walk home after about six weeks of treatment.
By 16 March 1867, when the first results of Lister's work were published in the Lancet, he had treated a total of eleven patients using his new antiseptic method. Of those, only one had died, and that was through a complication that was nothing to do with Lister's wound-dressing technique. Now, for the first time, patients with compound fractures were likely to leave the hospital with all their limbs intact :— Richard Hollingham, ''Blood and Guts: A History of Surgery'', p. 62
Before antiseptic operations were introduced at the hospital, there were sixteen deaths in thirty-five surgical cases. Almost one in every two patients died. After antiseptic surgery was introduced in the summer of 1865, there were only six deaths in forty cases. The mortality rate had dropped from almost 50 per cent to around 15 per cent. It was a remarkable achievement :— Richard Hollingham, ''Blood and Guts: A History of Surgery'', p. 63Phenol was the main ingredient of the
Carbolic Smoke Ball
''Carlill v Carbolic Smoke Ball Company'' 892EWCA Civ 1is an English contract law decision by the English Court of Appeal">Court of Appeal, which held an advertisement containing certain terms to get a reward constituted a binding unilateral of ...
, an ineffective device marketed in London in the 19th century as protection against influenza and other ailments, and the subject of the famous law case '' Carlill v Carbolic Smoke Ball Company''.
Second World War
The toxic effect of phenol on the central nervous system, discussed below, causes sudden collapse and loss of consciousness in both humans and animals; a state of cramping precedes these symptoms because of the motor activity controlled by the central nervous system. Injections of phenol were used as a means of individual execution by Nazi Germany during the Second World War.''The Experiments''by Peter Tyson. NOVA It was originally used by the Nazis in 1939 as part of the Aktion T4 euthanasia program.''The Nazi Doctors''
, Chapter 14, Killing with Syringes: Phenol Injections. By Dr. Robert Jay Lifton The Germans learned that extermination of smaller groups was more economical by injection of each victim with phenol. Phenol injections were given to thousands of people. Maximilian Kolbe was also killed with a phenol injection after surviving two weeks of dehydration and starvation in
Auschwitz
Auschwitz concentration camp ( (); also or ) was a complex of over 40 concentration and extermination camps operated by Nazi Germany in occupied Poland (in a portion annexed into Germany in 1939) during World War II and the Holocaust. It con ...
when he volunteered to die in place of a stranger
''A Stranger'' ( hr, Obrana i zaštita; ) is a 2013 Croatian drama film directed by Bobo Jelčić.
Cast
* Bogdan Diklić — Slavko
* Nada Đurevska — Milena
* Ivana Roščić — Zehra
* Rakan Rushaidat — Krešo
* Vinko Kraljević — Mil ...
. Approximately one gram is sufficient to cause death.
Occurrences
Phenol is a normal metabolic product, excreted in quantities up to 40 mg/L in human urine. The temporal gland secretion of male elephants showed the presence of phenol and4-methylphenol
''para''-Cresol, also 4-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of O-Cres ...
during musth.
It is also one of the chemical compounds found in castoreum. This compound is ingested from the plants the beaver eats.
Occurrence in whisky
Phenol is a measurable component in the aroma and taste of the distinctive Islay scotch whisky, generally ~30 ppm, but it can be over 160ppm in the malted barley used to produce whisky. This amount is different from and presumably higher than the amount in the distillate.Biodegradation
'' Cryptanaerobacter phenolicus'' is a bacterium species that producesbenzoate
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
from phenol via 4-hydroxybenzoate
4-Hydroxybenzoic acid, also known as ''p''-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar ...
. '' Rhodococcus phenolicus'' is a bacterium species able to degrade phenol as sole carbon source.
Toxicity
Phenol and its vapors are corrosive to the eyes, the skin, and the respiratory tract. Its corrosive effect on skin and mucous membranes is due to a protein-degenerating effect. Repeated or prolonged skin contact with phenol may cause dermatitis, or even second and third-degree burns. Inhalation of phenol vapor may cause lungedema
Edema, also spelled oedema, and also known as fluid retention, dropsy, hydropsy and swelling, is the build-up of fluid in the body's Tissue (biology), tissue. Most commonly, the legs or arms are affected. Symptoms may include skin which feels t ...
. The substance may cause harmful effects on the central nervous system and heart, resulting in dysrhythmia, seizures, and coma
A coma is a deep state of prolonged unconsciousness in which a person cannot be awakened, fails to respond normally to painful stimuli, light, or sound, lacks a normal wake-sleep cycle and does not initiate voluntary actions. Coma patients exhi ...
. The kidneys may be affected as well. Long-term or repeated exposure of the substance may have harmful effects on the liver and kidneys. There is no evidence that phenol causes cancer in humans. Besides its hydrophobic effects, another mechanism for the toxicity of phenol may be the formation of phenoxyl
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also kn ...
radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
.
Since phenol is absorbed through the skin relatively quickly, systemic poisoning can occur in addition to the local caustic burns. Resorptive poisoning by a large quantity of phenol can occur even with only a small area of skin, rapidly leading to paralysis of the central nervous system and a severe drop in body temperature. The for oral toxicity is less than 500 mg/kg for dogs, rabbits, or mice; the minimum lethal human dose was cited as 140 mg/kg. The Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services states the fatal dose for ingestion of phenol is from 1 to 32 g.
Chemical burns from skin exposures can be decontaminated by washing with polyethylene glycol, isopropyl alcohol, or perhaps even copious amounts of water. Removal of contaminated clothing is required, as well as immediate hospital
A hospital is a health care institution providing patient treatment with specialized health science and auxiliary healthcare staff and medical equipment. The best-known type of hospital is the general hospital, which typically has an emerge ...
treatment for large splashes. This is particularly important if the phenol is mixed with chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
(a commonly used mixture in molecular biology for DNA and RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
purification). Phenol is also a reproductive toxin causing increased risk of miscarriage and low birth weight indicating retarded development in utero.
Phenols
The word ''phenol'' is also used to refer to any compound that contains a six-membered aromatic ring, bonded directly to ahydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
(-OH). Thus, phenols are a class of organic compounds of which the phenol discussed in this article is the simplest member.
See also
*Bamberger rearrangement The Bamberger rearrangement is the chemical reaction of phenylhydroxylamines with strong aqueous acid, which will rearrange to give 4-aminophenols. It is named for the German chemist Eugen Bamberger (1857–1932).
The starting phenylhydroxylam ...
* Claisen rearrangement
* Cresol
*Fries rearrangement
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.
It involves migration of an acyl group of phenol ester to the aryl ...
* Polyphenol
References
External links
International Chemical Safety Card 0070
{{Authority control Antiseptics Commodity chemicals Hazardous air pollutants Oxoacids Phenyl compounds 1834 in science