Diamagnetic material interaction in magnetic field.png on:
[Wikipedia]
[Google]
[Amazon]
 Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an  Diamagnetism was first discovered when
Diamagnetism was first discovered when
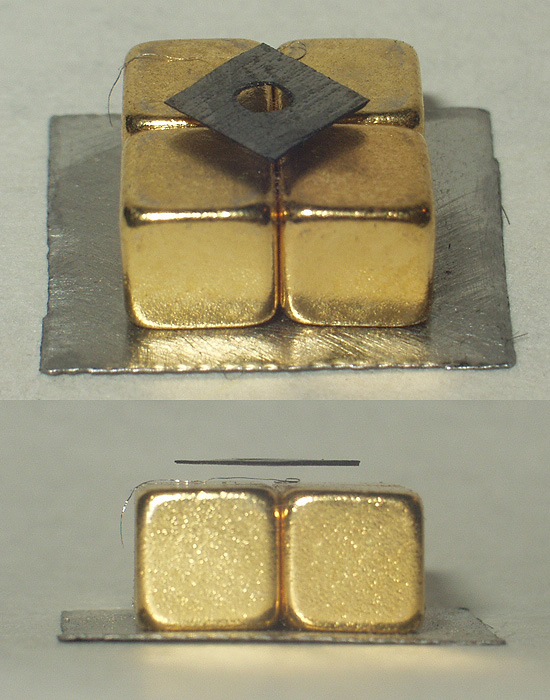
 Diamagnets may be levitated in stable equilibrium in a magnetic field, with no power consumption. Earnshaw's theorem seems to preclude the possibility of static magnetic levitation. However, Earnshaw's theorem applies only to objects with positive susceptibilities, such as ferromagnets (which have a permanent positive moment) and paramagnets (which induce a positive moment). These are attracted to field maxima, which do not exist in free space. Diamagnets (which induce a negative moment) are attracted to field minima, and there can be a field minimum in free space.
A thin slice of
Diamagnets may be levitated in stable equilibrium in a magnetic field, with no power consumption. Earnshaw's theorem seems to preclude the possibility of static magnetic levitation. However, Earnshaw's theorem applies only to objects with positive susceptibilities, such as ferromagnets (which have a permanent positive moment) and paramagnets (which induce a positive moment). These are attracted to field maxima, which do not exist in free space. Diamagnets (which induce a negative moment) are attracted to field minima, and there can be a field minimum in free space.
A thin slice of
Diamagnetic Levitation (YouTube)
Diamagnetism of water (YouTube, in Japanese)
{{Use dmy dates, date=May 2018 Electric and magnetic fields in matter Magnetic levitation Magnetism
 Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Dia ...
in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipole
In electromagnetism, a magnetic dipole is the limit of either a closed loop of electric current or a pair of poles as the size of the source is reduced to zero while keeping the magnetic moment constant. It is a magnetic analogue of the electric ...
s in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum
The vacuum magnetic permeability (variously ''vacuum permeability'', ''permeability of free space'', ''permeability of vacuum''), also known as the magnetic constant, is the magnetic permeability in a classical vacuum. It is a physical constant, ...
, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior.
 Diamagnetism was first discovered when
Diamagnetism was first discovered when Anton Brugmans
Anton Brugmans (1732–1789) was Dutch physicist who proposed a two-fluid theory of magnetism. He did magnetism experiments by putting objects on water or mercury, using surface tension to make them float and magnets to move them. He discovered th ...
observed in 1778 that bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
was repelled by magnetic fields. In 1845, Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic inducti ...
demonstrated that it was a property of matter and concluded that every material responded (in either a diamagnetic or paramagnetic way) to an applied magnetic field. On a suggestion by William Whewell
William Whewell ( ; 24 May 17946 March 1866) was an English polymath, scientist, Anglican priest, philosopher, theologian, and historian of science. He was Master of Trinity College, Cambridge. In his time as a student there, he achieved ...
, Faraday first referred to the phenomenon as ''diamagnetic'' (the prefix ''dia-'' meaning ''through'' or ''across''), then later changed it to ''diamagnetism''.
A simple rule of thumb is used in chemistry to determine whether a particle (atom, ion, or molecule) is paramagnetic or diamagnetic: If all electrons in the particle are paired, then the substance made of this particle is diamagnetic; If it has unpaired electrons, then the substance is paramagnetic.
Materials
Diamagnetism is a property of all materials, and always makes a weak contribution to the material's response to a magnetic field. However, other forms of magnetism (such asferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
or paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
) are so much stronger that, when multiple different forms of magnetism are present in a material, the diamagnetic contribution is usually negligible. Substances where the diamagnetic behaviour is the strongest effect are termed diamagnetic materials, or diamagnets. Diamagnetic materials are those that some people generally think of as ''non-magnetic'', and include water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, wood
Wood is a porous and fibrous structural tissue found in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulose fibers that are strong in tension and embedded in a matrix of lignin ...
, most organic compounds such as petroleum and some plastics, and many metals including copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, particularly the heavy ones with many core electrons, such as mercury, gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
and bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
. The magnetic susceptibility values of various molecular fragments are called Pascal's constants In magnetism, Pascals’ constants are numbers used in the evaluation of the magnetic susceptibilities of coordination compounds. The magnetic susceptibility of a compound is the sum of the paramagnetic susceptibility associated with the unpaired ...
.
Diamagnetic materials, like water, or water-based materials, have a relative magnetic permeability that is less than or equal to 1, and therefore a magnetic susceptibility less than or equal to 0, since susceptibility is defined as . This means that diamagnetic materials are repelled by magnetic fields. However, since diamagnetism is such a weak property, its effects are not observable in everyday life. For example, the magnetic susceptibility of diamagnets such as water is . The most strongly diamagnetic material is bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
, , although pyrolytic carbon
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.
Pyrolytic carbon is man-made and is thought not to be found in nature.Ratner, Buddy D. ...
may have a susceptibility of in one plane. Nevertheless, these values are orders of magnitude smaller than the magnetism exhibited by paramagnets and ferromagnets. Because ''χ''v is derived from the ratio of the internal magnetic field to the applied field, it is a dimensionless value.
In rare cases, the diamagnetic contribution can be stronger than paramagnetic contribution. This is the case for gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
, which has a magnetic susceptibility less than 0 (and is thus by definition a diamagnetic material), but when measured carefully with X-ray magnetic circular dichroism, has an extremely weak paramagnetic contribution that is overcome by a stronger diamagnetic contribution.
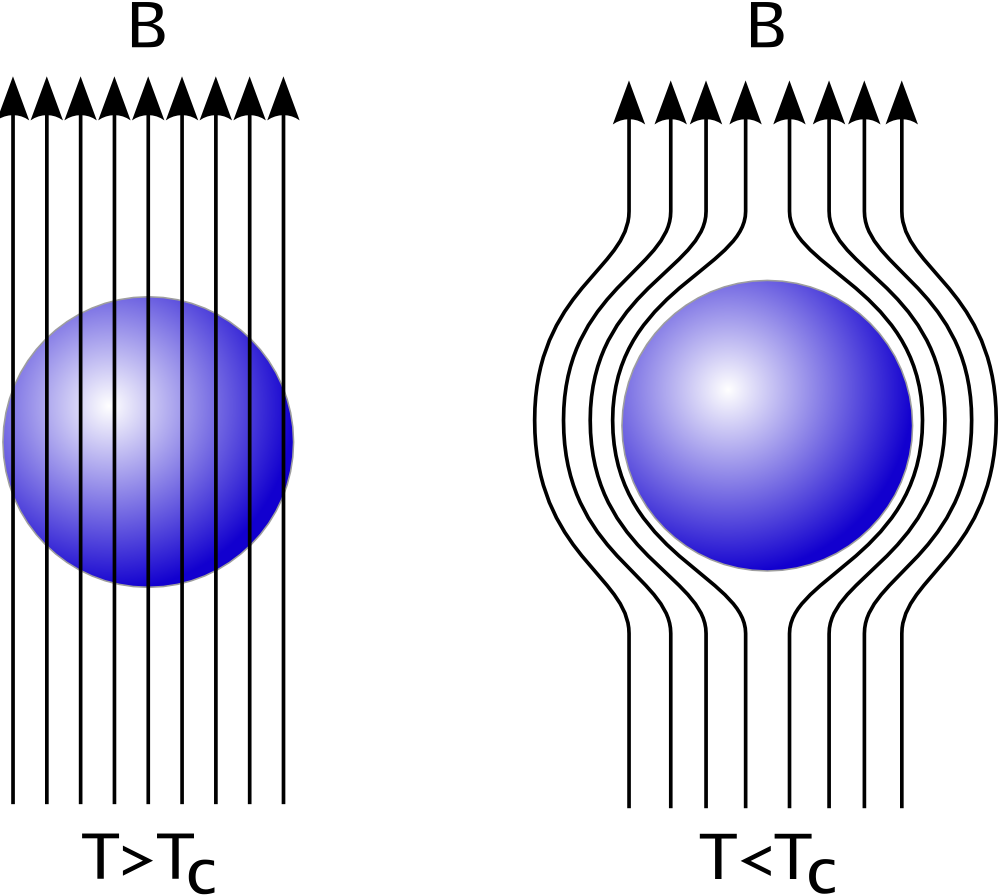
Superconductors

Superconductors
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
may be considered perfect diamagnets (), because they expel all magnetic fields (except in a thin surface layer) due to the Meissner effect
The Meissner effect (or Meissner–Ochsenfeld effect) is the expulsion of a magnetic field from a superconductor during its transition to the superconducting state when it is cooled below the critical temperature. This expulsion will repel a ne ...
.
Demonstrations
Curving water surfaces
If a powerful magnet (such as a supermagnet) is covered with a layer of water (that is thin compared to the diameter of the magnet) then the field of the magnet significantly repels the water. This causes a slight dimple in the water's surface that may be seen by a reflection in its surface.Levitation
 Diamagnets may be levitated in stable equilibrium in a magnetic field, with no power consumption. Earnshaw's theorem seems to preclude the possibility of static magnetic levitation. However, Earnshaw's theorem applies only to objects with positive susceptibilities, such as ferromagnets (which have a permanent positive moment) and paramagnets (which induce a positive moment). These are attracted to field maxima, which do not exist in free space. Diamagnets (which induce a negative moment) are attracted to field minima, and there can be a field minimum in free space.
A thin slice of
Diamagnets may be levitated in stable equilibrium in a magnetic field, with no power consumption. Earnshaw's theorem seems to preclude the possibility of static magnetic levitation. However, Earnshaw's theorem applies only to objects with positive susceptibilities, such as ferromagnets (which have a permanent positive moment) and paramagnets (which induce a positive moment). These are attracted to field maxima, which do not exist in free space. Diamagnets (which induce a negative moment) are attracted to field minima, and there can be a field minimum in free space.
A thin slice of pyrolytic graphite
Pyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.
Pyrolytic carbon is man-made and is thought not to be found in nature.Ratner, Buddy D. ...
, which is an unusually strongly diamagnetic material, can be stably floated in a magnetic field, such as that from rare earth permanent magnets. This can be done with all components at room temperature, making a visually effective and relatively convenient demonstration of diamagnetism.
The Radboud University Nijmegen
Radboud University (abbreviated as RU, nl, Radboud Universiteit , formerly ''Katholieke Universiteit Nijmegen'') is a public research university located in Nijmegen, the Netherlands. The university bears the name of Saint Radboud, a 9th century ...
, the Netherlands
)
, anthem = ( en, "William of Nassau")
, image_map =
, map_caption =
, subdivision_type = Sovereign state
, subdivision_name = Kingdom of the Netherlands
, established_title = Before independence
, established_date = Spanish Netherl ...
, has conducted experiments where water and other substances were successfully levitated. Most spectacularly, a live frog (see figure) was levitated.
In September 2009, NASA's Jet Propulsion Laboratory
The Jet Propulsion Laboratory (JPL) is a federally funded research and development center and NASA field center in the City of La Cañada Flintridge, California, United States.
Founded in the 1930s by Caltech researchers, JPL is owned by NASA an ...
(JPL) in Pasadena, California announced it had successfully levitated mice using a superconducting magnet, an important step forward since mice are closer biologically to humans than frogs. JPL said it hopes to perform experiments regarding the effects of microgravity on bone and muscle mass.
Recent experiments studying the growth of protein crystals have led to a technique using powerful magnets to allow growth in ways that counteract Earth's gravity.
A simple homemade device for demonstration can be constructed out of bismuth plates and a few permanent magnets that levitate a permanent magnet.
Theory
The electrons in a material generally settle in orbitals, with effectively zero resistance and act like current loops. Thus it might be imagined that diamagnetism effects in general would be common, since any applied magnetic field would generate currents in these loops that would oppose the change, in a similar way to superconductors, which are essentially perfect diamagnets. However, since the electrons are rigidly held in orbitals by the charge of the protons and are further constrained by thePauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulat ...
, many materials exhibit diamagnetism, but typically respond very little to the applied field.
The Bohr–Van Leeuwen theorem proves that there cannot be any diamagnetism or paramagnetism in a purely classical system. However, the classical theory of Langevin for diamagnetism gives the same prediction as the quantum theory. The classical theory is given below.
Langevin diamagnetism
Paul Langevin
Paul Langevin (; ; 23 January 1872 – 19 December 1946) was a French physicist who developed Langevin dynamics and the Langevin equation. He was one of the founders of the ''Comité de vigilance des intellectuels antifascistes'', an an ...
's theory of diamagnetism (1905) applies to materials containing atoms with closed shells (see dielectrics). A field with intensity , applied to an electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
with charge and mass , gives rise to Larmor precession
In physics, Larmor precession (named after Joseph Larmor) is the precession of the magnetic moment of an object about an external magnetic field. The phenomenon is conceptually similar to the precession of a tilted classical gyroscope in an extern ...
with frequency . The number of revolutions per unit time is , so the current for an atom with electrons is (in SI units)
:
The magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagne ...
of a current loop is equal to the current times the area of the loop. Suppose the field is aligned with the axis. The average loop area can be given as , where is the mean square distance of the electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
perpendicular to the axis. The magnetic moment is therefore
:
If the distribution of charge is spherically symmetric, we can suppose that the distribution of coordinates are independent and identically distributed
In probability theory and statistics, a collection of random variables is independent and identically distributed if each random variable has the same probability distribution as the others and all are mutually independent. This property is usual ...
. Then , where is the mean square distance of the electrons from the nucleus. Therefore, . If is the number of atoms per unit volume, the volume diamagnetic susceptibility in SI units is
:
In atoms, Langevin susceptibility is of the same order of magnitude as Van Vleck paramagnetic susceptibility.
In metals
The Langevin theory is not the full picture formetals
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typical ...
because there are also non-localized electrons. The theory that describes diamagnetism in a free electron gas Free electron in physics may refer to:
*Electron, as a free particle
*Solvated electron
*Charge carrier, as carriers of electric charge
*Valence electron, as an outer shell electron that is associated with an atom
*Valence and conduction bands, as a ...
is called Landau diamagnetism, named after Lev Landau
Lev Davidovich Landau (russian: Лев Дави́дович Ланда́у; 22 January 1908 – 1 April 1968) was a Soviet-Azerbaijani physicist of Jewish descent who made fundamental contributions to many areas of theoretical physics.
His ac ...
, and instead considers the weak counteracting field that forms when the electrons' trajectories are curved due to the Lorentz force. Landau diamagnetism, however, should be contrasted with Pauli paramagnetism, an effect associated with the polarization of delocalized electrons' spins. For the bulk case of a 3D system and low magnetic fields, the (volume) diamagnetic susceptibility can be calculated using Landau quantization In quantum mechanics, Landau quantization refers to the quantization of the cyclotron orbits of charged particles in a uniform magnetic field. As a result, the charged particles can only occupy orbits with discrete, equidistant energy values, call ...
, which in SI units is
:
where is the Fermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy difference between the highest and lowest occupied single-particle states in a quantum system of non-interacting fermions at absolute zero temperature.
In a Fermi ga ...
. This is equivalent to , exactly times Pauli paramagnetic susceptibility, where is the Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
The Bohr magneton, in SI units is defined as
\mu_\mat ...
and is the density of states
In solid state physics and condensed matter physics, the density of states (DOS) of a system describes the number of modes per unit frequency range. The density of states is defined as D(E) = N(E)/V , where N(E)\delta E is the number of states i ...
(number of states per energy per volume). This formula takes into account the spin degeneracy of the carriers (spin ½ electrons).
In doped semiconductors the ratio between Landau and Pauli susceptibilities may change due to the effective mass of the charge carriers differing from the electron mass in vacuum, increasing the diamagnetic contribution. The formula presented here only applies for the bulk; in confined systems like quantum dots, the description is altered due to quantum confinement
A potential well is the region surrounding a local minimum of potential energy. Energy captured in a potential well is unable to convert to another type of energy (kinetic energy in the case of a gravitational potential well) because it is capt ...
. Additionally, for strong magnetic fields, the susceptibility of delocalized electrons oscillates as a function of the field strength, a phenomenon known as the De Haas–Van Alphen effect, also first described theoretically by Landau.
See also
*Antiferromagnetism
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
*Magnetochemistry
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaire ...
* Moses effect
*
References
External links
*Diamagnetic Levitation (YouTube)
Diamagnetism of water (YouTube, in Japanese)
{{Use dmy dates, date=May 2018 Electric and magnetic fields in matter Magnetic levitation Magnetism