Electrophilic halogenation on:
[Wikipedia]
[Google]
[Amazon]
In 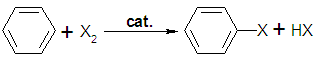 A few types of aromatic compounds, such as
A few types of aromatic compounds, such as
 The mechanism for iodination is slightly different:
The mechanism for iodination is slightly different:
 However, if a catalyst is used with excess bromine, then a tribromide will be formed.
Halogenation of phenols is faster in polar solvents in a basic environment due to the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
Chlorination of
However, if a catalyst is used with excess bromine, then a tribromide will be formed.
Halogenation of phenols is faster in polar solvents in a basic environment due to the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
Chlorination of  No reaction takes place when the solvent is replaced by tetrachloromethane. In contrast, when the reactant is
No reaction takes place when the solvent is replaced by tetrachloromethane. In contrast, when the reactant is  The food dye erythrosine can be synthesized by
The food dye erythrosine can be synthesized by  This reaction is driven by
This reaction is driven by
Abstract
/ref>
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
. This organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
is typical of aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
compounds and a very useful method for adding substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as '' side ...
s to an aromatic system.
:phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
, will react without a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, but for typical benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
derivatives with less reactive substrates, a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
is required as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. Typical Lewis acid catalysts include , , and . These work by forming a highly electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
complex which is attacked by the benzene ring.
Reaction mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
for chlorination of benzene is the same as bromination of benzene. Iron(III) bromide
Iron(III) bromide is the chemical compound with the formula FeBr3. Also known as ferric bromide, this red-brown odorless compound is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It dissolves in water to give acidic s ...
and iron(III) chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The col ...
become inactivated if they react with water, including moisture in the air. Therefore, they are generated by adding iron filings to bromine or chlorine. Here is the mechanism of this reaction:
: The mechanism for iodination is slightly different:
The mechanism for iodination is slightly different: iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
(I2) is treated with an oxidizing agent such as nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
to obtain the electrophilic iodine ("I+", probably IONO2). Other conditions for iodination include I2, HIO3, H2SO4, and ''N''-iodosuccinimide, H2SO4. These conditions are successful for highly deactivated arenes, including nitroaromatics.
In a series of studies, the powerful reagent obtained by using a mixture of iodine and potassium iodate
Potassium iodate ( K I O3) is an ionic chemical compound consisting of K+ ions and IO3− ions in a 1:1 ratio.
Preparation and properties
Potassium iodate is an oxidizing agent and as such it can cause fires if in contact with combustible mater ...
dissolved in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
was used. Here the iodinating agent is the triiodine cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
I3+ and the base is HSO4−. In these studies both the kinetics of the reaction and the preparative conditions for the iodination of strongly deactivated compounds, such as benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin ...
and 3-nitrobenzotrifluoride, were investigated.
While electrophilic fluorination is possible with F2/N2 (10%), XeF2, or N-F reagents like Selectfluor
Selectfluor, a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the nucleophillic base DABCO. It is a colourless salt that tolerates air and even water. It has be ...
, these methods are seldom used, due to the formation of isomeric mixtures and polyfluorination products. Although mixtures also form in the case of other aromatic halogenations, fluoroaromatics are often extremely challenging to separate from their nonfluorinated, polyfluorinated, and/or isomeric counterparts.
The initial step of the halogenation of aromatic compounds differs from that of the halogenation of alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
in that alkenes do not require a catalyst to enhance the electrophilicity of the halogen. The formation of the arenium ion
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution.
For historic reasons this complex is also called a Wheland intermediate, after American chemist George ...
results in the temporary loss of aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
, which has a higher activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
compared to halonium ion formation in alkenes. In other words, alkenes are more reactive and do not need to have the Br–Br or Cl–Cl bond weakened.
Scope
If the ring contains a strongly activating substituent such as –OH, –OR oramine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
s, a catalyst is not necessary, for example in the bromination of ''p''-cresol:
: However, if a catalyst is used with excess bromine, then a tribromide will be formed.
Halogenation of phenols is faster in polar solvents in a basic environment due to the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
Chlorination of
However, if a catalyst is used with excess bromine, then a tribromide will be formed.
Halogenation of phenols is faster in polar solvents in a basic environment due to the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
Chlorination of toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
with chlorine without catalyst requires a polar solvent as well such as acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
. The ''ortho'' to ''para'' selectivity is low:
: No reaction takes place when the solvent is replaced by tetrachloromethane. In contrast, when the reactant is
No reaction takes place when the solvent is replaced by tetrachloromethane. In contrast, when the reactant is 2-phenylethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace ami ...
, it is possible to employ relatively apolar solvents with exclusive '' ortho-'' regioselectivity due to the intermediate formation of a chloramine
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
, enabling the Intramolecular reaction.
: The food dye erythrosine can be synthesized by
The food dye erythrosine can be synthesized by iodination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers ...
of another dye called fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used ...
:
: This reaction is driven by
This reaction is driven by sodium bicarbonate
Sodium bicarbonate ( IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3− ...
."Synthesis of Triarylmethane and Xanthene Dyes Using Electrophilic Aromatic Substitution Reactions" James V. McCullagh and Kelly A. Daggett '' J. Chem. Educ.'' 2007, 84, 1799Abstract
/ref>
See also
*Electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
* Electrophilic fluorination
* Halogenation reactions
References
{{DEFAULTSORT:Electrophilic Halogenation Halogenation reactions