Diuranate on:
[Wikipedia]
[Google]
[Amazon]
 A uranate is a
A uranate is a

 All uranates(VI) are mixed oxides, that is, compounds made up of metal(s), uranium and oxygen atoms. No uranium
All uranates(VI) are mixed oxides, that is, compounds made up of metal(s), uranium and oxygen atoms. No uranium 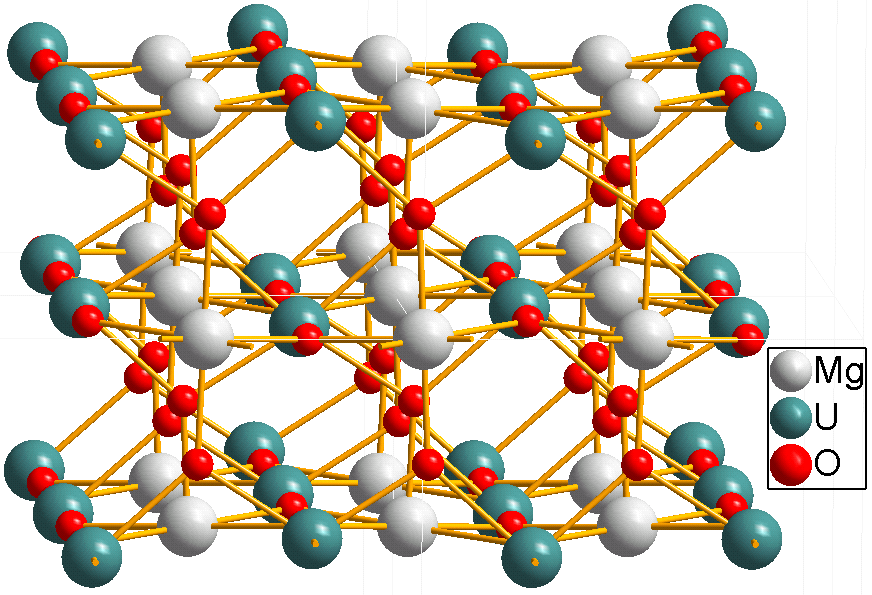 Magnesium uranate, MgUO4, has a quite different structure. Distorted UO6 octahedra are linked into infinite chains; the "uranyl" U-O bond length is 192 pm, not much shorter than the other U-O bond length of 218 pm.
A number of so-called diuranates are known. They fall into two categories, compounds of exact composition, synthesized by combination of metal oxides or thermal decomposition of salts of uranyl complexes and substances of approximate composition, found in
Magnesium uranate, MgUO4, has a quite different structure. Distorted UO6 octahedra are linked into infinite chains; the "uranyl" U-O bond length is 192 pm, not much shorter than the other U-O bond length of 218 pm.
A number of so-called diuranates are known. They fall into two categories, compounds of exact composition, synthesized by combination of metal oxides or thermal decomposition of salts of uranyl complexes and substances of approximate composition, found in

 ADU is an intermediate in the production of uranium oxides to be used as
ADU is an intermediate in the production of uranium oxides to be used as
 A uranate is a
A uranate is a ternary
Ternary (from Latin ''ternarius'') or trinary is an adjective meaning "composed of three items". It can refer to:
Mathematics and logic
* Ternary numeral system, a base-3 counting system
** Balanced ternary, a positional numeral system, useful ...
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
involving the element uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
in one of the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s 4, 5 or 6. A typical chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
is MxUyOz, where M represents a cation. The uranium atom in uranates(VI) has two short collinear U–O bonds and either four or six more next nearest oxygen atoms. The structures are infinite lattice structures with the uranium atoms linked by bridging oxygen atoms.
Uranium oxides are the foundation of the nuclear fuel cycle (" ammonium diuranate" and "sodium diuranate
Sodium diuranate, Na2U2O7·6H2O, is a uranium salt also known as the yellow oxide of uranium. Sodium diuranate is commonly referred to by the initials SDU. Along with ammonium diuranate it was a component in early yellowcakes. The ratio of the tw ...
" are intermediates in the production of uranium oxide nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
s) and their long-term geological disposal requires a thorough understanding of their chemical reactivity, phase transitions, and physical and chemical properties.
Synthesis
A method of general applicability involves combining two oxides in a high temperature reaction. For example, :Na2O + UO3 → Na2UO4 Another method is the thermal decomposition of a complex, such as anacetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
complex. For example, microcrystalline barium diuranate, BaU2O7, was made by thermal decomposition of barium uranyl acetate at 900 °C.
:Ba O2(ac)3sub>2 → BaU2O7 ... (ac=CH3CO2−)
Uranates can be prepared by adding alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
to an aqueous solution of a uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula . It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may ...
salt. However, the composition of the precipitate that forms is variable and depends on the chemical and physical conditions used.
Uranates are insoluble in water and other solvents, so pure samples can only be obtained by careful control of reaction conditions.
Uranium(VI)
Structures

 All uranates(VI) are mixed oxides, that is, compounds made up of metal(s), uranium and oxygen atoms. No uranium
All uranates(VI) are mixed oxides, that is, compounds made up of metal(s), uranium and oxygen atoms. No uranium oxyanion An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determine ...
, such as O4sup>2− or 2O7sup>2−, is known. Instead, all uranate structures are based on UOn polyhedra sharing oxygen atoms in an infinite lattice. The structures of uranates(VI) are unlike the structure of any mixed oxide of elements other than actinide elements. A particular feature is the presence of linear O-U-O moieties, which resemble the uranyl
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula . It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may ...
ion, UO22+. However, the U-O bond length varies from 167 pm, which is similar to the bond length of the uranyl ion, up to about 208 pm in the related compound α-UO3, so it is debatable as to whether these compounds all contain the uranyl ion. There are two principal types of uranate which are defined by the number of nearest-neighbour oxygen atoms in addition to the "uranyl" oxygens.
In one group, including M2UO4 (M=Li, Na, K) and MUO4 (M=Ca, Sr) there are six additional oxygen atoms. Taking calcium uranate, CaUO4, as an example, the six oxygen atoms are arranged as a flattened octahedron
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
, flattened along the 3-fold symmetry axis
Rotational symmetry, also known as radial symmetry in geometry, is the property a shape has when it looks the same after some rotation by a partial turn. An object's degree of rotational symmetry is the number of distinct orientations in which i ...
of the octahedron which also runs through the O-U-O axis (local point group
In geometry, a point group is a mathematical group of symmetry operations (isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every p ...
D3d at the uranium atom). Each of these oxygen atoms is shared between three uranium atoms, which accounts for the stoichiometry, U 2×O 6×1/3 O = UO4. The structure has been described as a hexagonal layer structure. It can also viewed as a distorted fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs sca ...
structure in which two U-O distances have decreased and the other six have increased.
In the other group, exemplified by barium uranate, BaUO4, there are four additional oxygen atoms. These four oxygens lie in a plane and each is shared between two uranium atoms, which accounts for the stoichiometry, U 2×O 4×1/2 O = UO4. The structure may called a tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square ...
layer structure.
 Magnesium uranate, MgUO4, has a quite different structure. Distorted UO6 octahedra are linked into infinite chains; the "uranyl" U-O bond length is 192 pm, not much shorter than the other U-O bond length of 218 pm.
A number of so-called diuranates are known. They fall into two categories, compounds of exact composition, synthesized by combination of metal oxides or thermal decomposition of salts of uranyl complexes and substances of approximate composition, found in
Magnesium uranate, MgUO4, has a quite different structure. Distorted UO6 octahedra are linked into infinite chains; the "uranyl" U-O bond length is 192 pm, not much shorter than the other U-O bond length of 218 pm.
A number of so-called diuranates are known. They fall into two categories, compounds of exact composition, synthesized by combination of metal oxides or thermal decomposition of salts of uranyl complexes and substances of approximate composition, found in yellowcake
Yellowcake (also called urania) is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before f ...
. The name refers only to the empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is th ...
, MxU2O7; the structures are completely different from ions such as the dichromate ion. For example, in barium diuranate, BaU2O7, UO6 octahedral units are joined by sharing edges, forming infinite chains in the directions of the crystallographic ''a'' and ''b'' directions.
Uranates with more complicated empirical formulas are known. Essentially these arise when the cation:uranium ratio is different from 2:1 (monovalent cations) or 1:1 (divalent cations). Charge-balance constrains the number of oxygen atoms to be equal to half the sum of charges of the cations and uranyl groups. For example, with the cation K+, compounds with K:U ratios of 2, 1 and 0.5 were found, corresponding to empirical formulas K2UO4, K2U2O7 and K2U4O13. The uranate structures in these compounds differ in the way the UOx structural units are linked together.
Properties and uses

Yellowcake
Yellowcake (also called urania) is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before f ...
is produced in the separation of uranium from other elements, by adding alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
to a solution containing uranyl salts.
When the alkali used is ammonia, so-called ammonium diuranate, known in the industry as ADU, is the main constituent of yellowcake. The exact composition of the precipitate depends to some extent on the conditions and anions that are present and the formula (NH4)2U2O7, is only an approximation. The precipitates obtained on addition of ammonia to uranyl nitrate solution under different conditions of temperature and final pH, when dried, were considered as loosely bound compounds with an ammonia/uranium ratio of 0.37 containing varying amounts of water and ammonium nitrate
Ammonium nitrate is a chemical compound with the chemical formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is ...
. In other studies it was found to approximate to the gross formulas 3UO3·NH3·5H2O,
The asymmetric stretching frequency of the uranyl ion was found to decrease with increasing NH4+ content. This decrease is continuous and no band splitting was observed, indicating that the ammonium uranate system is homogeneous and continuous.
 ADU is an intermediate in the production of uranium oxides to be used as
ADU is an intermediate in the production of uranium oxides to be used as nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
; it is converted directly into an oxide by heating. β-UO3 is produced at about 350 °C and U3O8 is obtained at higher temperatures. When the alkali used is sodium hydroxide, so-called sodium diuranate, SDU, is produced. This can also be converted into an oxide. Another choice of alkali is magnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ...
, making magnesium diuranate, known as MDU.
Oxides and uranates of uranium(VI) have been used in the past as yellow ceramic glazes as in Fiesta and to make yellow-green uranium glass
Uranium glass is glass which has had uranium, usually in oxide diuranate form, added to a glass mix before melting for colouration. The proportion usually varies from trace levels to about 2% uranium by weight, although some 20th-century piec ...
. Both of these applications are abandoned due to concern regarding radioactivity of the uranium. Uranates are important in radioactive waste management.
Uranium(V)
Several series of uranates(V) have been characterized. Compounds with the formula MIUO3 have aperovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known as ...
structure. Compounds MI3UO4 have a defect rock-salt structure
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pi ...
. MI7UO6 structures are based on a hexagonally close-packed array of oxygen atoms. In all cases the uranium is at the centre of an octahedron
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
of oxygen atoms. Also MIIIUO4 have been recently synthesized and characterized (MIII=Bi, Fe, Cr etc.). Few other compounds of uranium(V) are stable.
Uranium(IV)
Barium uranate, BaUO3, is made frombarium oxide
Barium oxide, also known as baria, is a white hygroscopic non-flammable compound with the formula BaO. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and if swallowed in large q ...
and uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear rea ...
in an atmosphere that contains absolutely no oxygen. It has a cubic crystal structure (space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
Pmm).
References
Further reading
*{{cite book , last1=Burns , first1=C. J. , last2=Neu , first2=M. P. , last3=Boukhalfa , first3=H. , last4=Gutowski , first4=K. E. , last5=Bridges , first5=N. J. , last6=Roger , first6=R. D. , title=Comprehensive Coordination Chemistry II , year=2004 , publisher=Elsevier , isbn=978-0-08-043748-4 , pages=189–345 , chapter=Chapter 3.3, The Actinides , doi=10.1016/B0-08-043748-6/02001-6 Uranates