Dissolved oxygen on:
[Wikipedia]
[Google]
[Amazon]
 Oxygen saturation (symbol SO2) is a relative measure of the concentration of
Oxygen saturation (symbol SO2) is a relative measure of the concentration of
 In aquatic environments, oxygen saturation is a ratio of the concentration of "dissolved
In aquatic environments, oxygen saturation is a ratio of the concentration of "dissolved
 Oxygen saturation (symbol SO2) is a relative measure of the concentration of
Oxygen saturation (symbol SO2) is a relative measure of the concentration of oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium at the given temperature. It can be measured with a dissolved oxygen probe such as an oxygen sensor or an optode in liquid media, usually water. The standard unit of oxygen saturation is percent (%).
Oxygen saturation can be measured regionally and noninvasively. Arterial oxygen saturation (SaO2) is commonly measured using pulse oximetry. Tissue saturation at peripheral scale can be measured using NIRS. This technique can be applied on both muscle and brain.
In medicine
Inmedicine
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
, oxygen saturation refers to ''oxygenation'', or when oxygen molecules () enter the tissues of the body. In this case blood is oxygenated in the lung
The lungs are the primary Organ (biology), organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the Vertebral column, backbone on either side of the heart. Their ...
s, where oxygen molecules travel from the air into the blood. Oxygen saturation (() sats) measures the percentage of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
binding sites in the bloodstream occupied by oxygen. Fish, invertebrates, plants, and aerobic bacteria all require oxygen.
In environmental science
 In aquatic environments, oxygen saturation is a ratio of the concentration of "dissolved
In aquatic environments, oxygen saturation is a ratio of the concentration of "dissolved oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
" (DO, O2), to the maximum amount of oxygen that will dissolve in that water body, at the temperature and pressure which constitute stable equilibrium conditions. Well-aerated water (such as a fast-moving stream) without oxygen producers or consumers is 100% saturated.
Stagnant water can become somewhat supersaturated
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a ...
with oxygen (i.e., reach more than 100% saturation) either because of the presence of photosynthetic aquatic oxygen producers or because of a slow equilibration after a change of atmospheric conditions. Stagnant water in the presence of decaying matter will typically have an oxygen concentration much less than 100%, which is due to anaerobic bacteria being much less efficient at breaking down organic material. Similarly as in water, oxygen concentration also plays a key role in the breakdown of organic matter in soils. Higher oxygen saturation allows aerobic bacteria to persist, which breaks down decaying organic material in soils much more efficiently than anaerobic bacteria. Thus, soils with high oxygen saturation will have less organic matter per volume than those with low oxygen saturation.
Environmental oxygenation can be important to the sustainability
Sustainability is a social goal for people to co-exist on Earth over a long period of time. Definitions of this term are disputed and have varied with literature, context, and time. Sustainability usually has three dimensions (or pillars): env ...
of a particular ecosystem
An ecosystem (or ecological system) is a system formed by Organism, organisms in interaction with their Biophysical environment, environment. The Biotic material, biotic and abiotic components are linked together through nutrient cycles and en ...
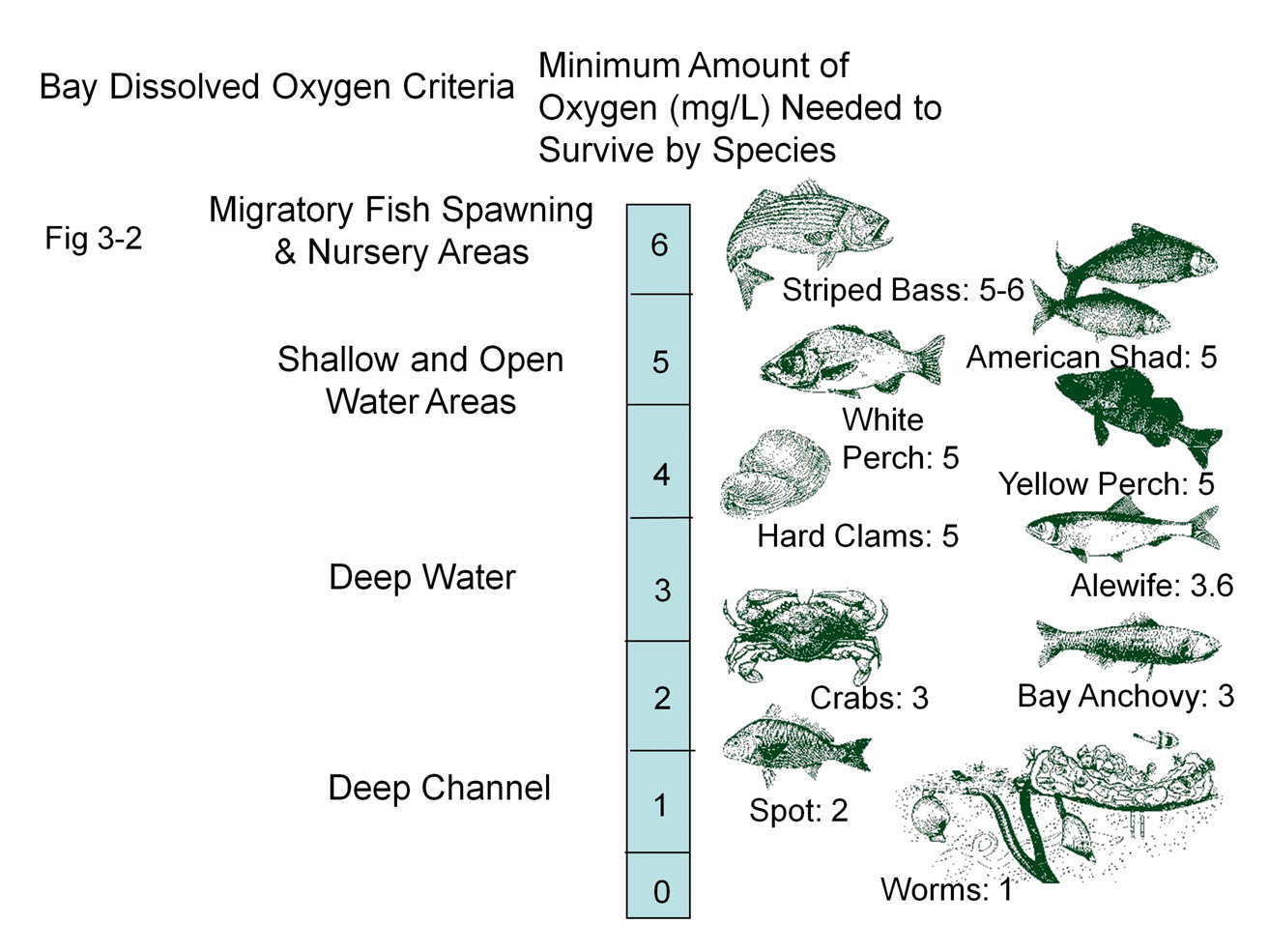
. The US Environmental Protection Agency has published a table of maximum equilibrium dissolved oxygen concentration versus temperature at atmospheric pressure. The optimal levels in an estuary for dissolved oxygen is higher than six ppm. Insufficient oxygen ( environmental hypoxia), often caused by the decomposition of organic matter and nutrient pollution
Nutrient pollution is a form of water pollution caused by too many Nutrient, nutrients entering the water. It is a primary cause of eutrophication of surface waters (lakes, rivers and Coast, coastal waters), in which excess nutrients, usually ni ...
, may occur in bodies of water such as pond
A pond is a small, still, land-based body of water formed by pooling inside a depression (geology), depression, either naturally or artificiality, artificially. A pond is smaller than a lake and there are no official criteria distinguishing ...
s and river
A river is a natural stream of fresh water that flows on land or inside Subterranean river, caves towards another body of water at a lower elevation, such as an ocean, lake, or another river. A river may run dry before reaching the end of ...
s, tending to suppress the presence of aerobic organism
An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment. The ability to exhibit aerobic respiration may yield benefits to the aerobic organism, as aerobic respiration yields more energy than anaerobic ...
s such as fish
A fish (: fish or fishes) is an aquatic animal, aquatic, Anamniotes, anamniotic, gill-bearing vertebrate animal with swimming fish fin, fins and craniate, a hard skull, but lacking limb (anatomy), limbs with digit (anatomy), digits. Fish can ...
. Deoxygenation increases the relative population of anaerobic organism
An anaerobic organism or anaerobe is any organism that does not require oxygen, molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an o ...
s such as plants and some bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
, resulting in fish kills and other adverse events. The net effect is to alter the balance of nature by increasing the concentration of anaerobic over aerobic species
A species () is often defined as the largest group of organisms in which any two individuals of the appropriate sexes or mating types can produce fertile offspring, typically by sexual reproduction. It is the basic unit of Taxonomy (biology), ...
.
See also
*Oxygen deficiency
Hypoxia is a condition in which the body or a region of the body is deprived of an adequate oxygen supply at the tissue level. Hypoxia may be classified as either '' generalized'', affecting the whole body, or ''local'', affecting a region of t ...
References
{{Wastewater Aquatic ecology Blood Water quality indicators Oxygen