Diagonal relationship on:
[Wikipedia]
[Google]
[Amazon]
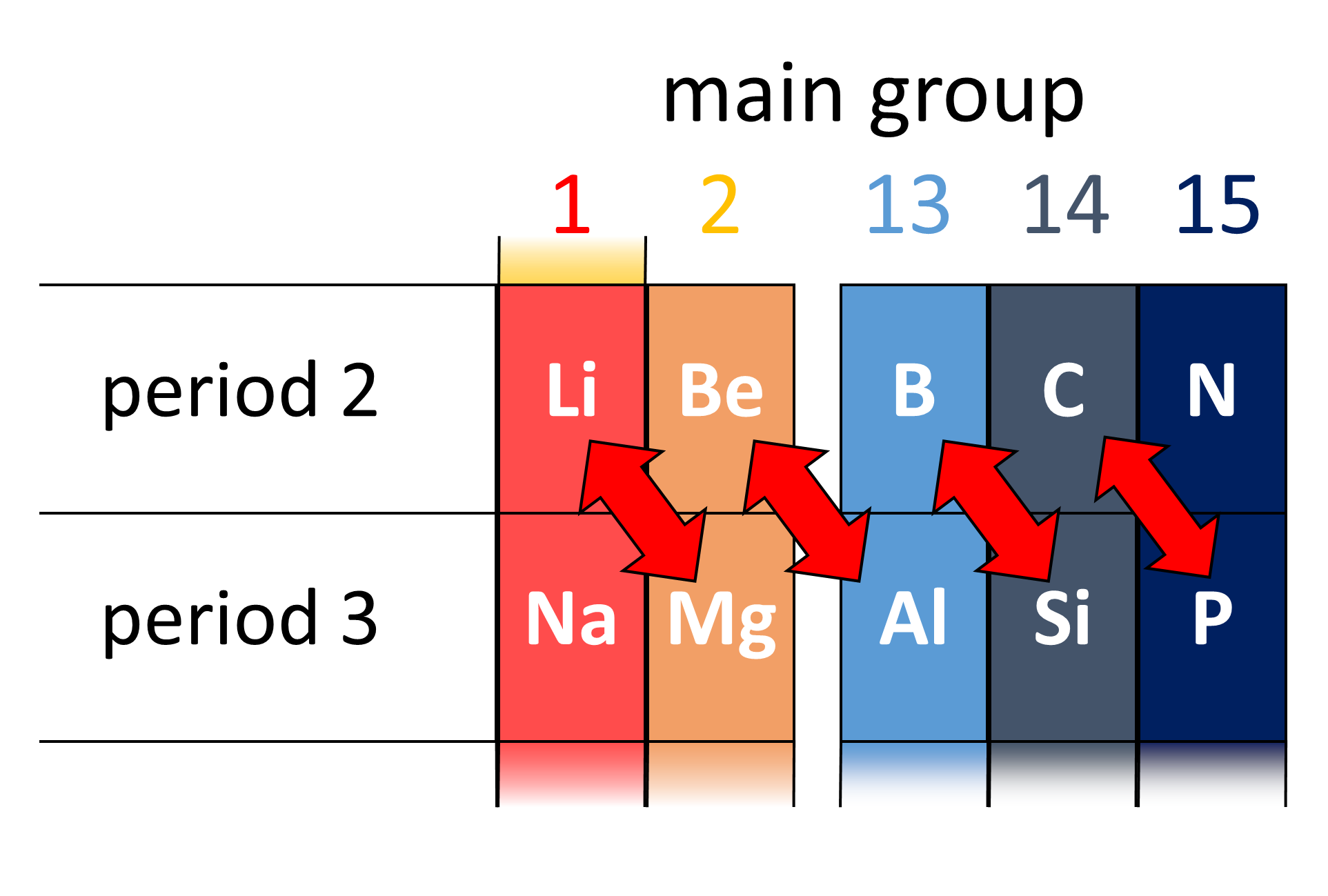 A diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods (first 20 elements) of the
A diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods (first 20 elements) of the
 A diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods (first 20 elements) of the
A diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods (first 20 elements) of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. These pairs (lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense soli ...
(Li) and magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
(Mg), beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
(Be) and aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
(Al), boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
(B) and silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
(Si), etc.) exhibit similar properties; for example, boron and silicon are both semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way ...
s, forming halides that are hydrolysed in water and have acidic oxides.
The organization of elements on the periodic table into horizontal rows and vertical columns makes certain relationships more apparent (periodic law
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
). Moving rightward and descending the periodic table have opposite effects on atomic radii
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
of isolated atoms. Moving rightward across the period decreases the atomic radii of atoms, while moving down the group will increase the atomic radii.
Similarly, on moving rightward a period, the elements become progressively more covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
, less basic and more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, whereas on moving down a group the elements become more ionic, more basic and less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
. Thus, on both descending a period ''and'' crossing a group by one element, the changes "cancel" each other out, and elements with similar properties which have similar chemistry are often found – the atomic size, electronegativity, properties of compounds (and so forth) of the diagonal members are similar.
It is found that the chemistry of a period 2 element
A period 2 element is one of the chemical elements in the second row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of th ...
often has similarities to the chemistry of the period 3 element one column to the right of it in the periodic table. Thus, the chemistry of Li has similarities to that of Mg, the chemistry of Be has similarities to that of Al, and the chemistry of B has similarities to that of Si. These are called diagonal relationships. (They are not as noticeable after B and Si.
The reasons for the existence of diagonal relationships are not fully understood, but charge density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system i ...
is a factor. For example, Li+ is a small cation with a +1 charge and Mg2+ is somewhat larger with a +2 charge, so the ionic potential of each of the two ions is roughly the same. It was revealed by an examination that the charge density of lithium is much closer to that of magnesium than to those of the other alkali metals.
Using the Li–Mg pair (under room temperature and pressure):
# When combined with oxygen under standard conditions, Li and Mg form only normal oxides whereas Na forms peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
and metals below Na, in addition, form superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
s.
# Li is the only group 1 element which forms a stable nitride
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resistant ...
, Li3N. Mg, as well as other group 2 elements, also form nitrides.
# Lithium carbonate, phosphate and fluoride are sparingly soluble in water. The corresponding group 2 salts are insoluble. (Think lattice and solvation energies).
# Both Li and Mg form covalent organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
compounds. LiMe and MgMe2 (cf. Grignard reagents
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
) are both valuable synthetic reagents. The other group 1 and group 2 analogues are ionic and extremely reactive (and hence difficult to manipulate).
# Chlorides of both Li and Mg are deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance' ...
(absorb moisture from surroundings) and soluble in alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
and pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
. Lithium chloride, like magnesium chloride (MgCl2·6H2O) separates out from hydrated crystal LiCl·2H2O.
#Lithium carbonate
Lithium carbonate is an inorganic compound, the lithium salt of carbonate with the formula . This white salt is widely used in the processing of metal oxides. It is listed on the World Health Organization's List of Essential Medicines because it c ...
and magnesium carbonate
Magnesium carbonate, (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.
Forms
The most common magnesium carbonate forms are ...
are both unstable and can produce corresponding oxides and carbon dioxide when they are heated.
Further diagonal similarities have also been suggested for carbon-phosphorus and nitrogen-sulfur, along with extending the Li-Mg and Be-Al relationships down into the transition elements (such as scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
).
References