DNA replication on:
[Wikipedia]
[Google]
[Amazon]
 In
In


 DNA replication, like all biological polymerization processes, proceeds in three enzymatically catalyzed and coordinated steps: initiation, elongation and termination.
DNA replication, like all biological polymerization processes, proceeds in three enzymatically catalyzed and coordinated steps: initiation, elongation and termination.

 For a cell to divide, it must first replicate its DNA. DNA replication is an all-or-none process; once replication begins, it proceeds to completion. Once replication is complete, it does not occur again in the same cell cycle. This is made possible by the division of initiation of th
For a cell to divide, it must first replicate its DNA. DNA replication is an all-or-none process; once replication begins, it proceeds to completion. Once replication is complete, it does not occur again in the same cell cycle. This is made possible by the division of initiation of th
pre-replication complex
An essential Noc3p dimerization cycle mediates ORC double-hexamer formation in replication licensing
ORC and Noc3p are continuously bound to the chromatin throughout the cell cycle. Cdc6 and Cdt1 then associate with the bound origin recognition complex at the origin in order to form a larger complex necessary to load the Mcm complex onto the DNA. In eukaryotes, the Mcm complex is the helicase that will split the DNA helix at the replication forks and origins. The Mcm complex is recruited at late G1 phase and loaded by the ORC-Cdc6-Cdt1 complex onto the DNA via ATP-dependent protein remodeling. The loading of the MCM complex onto the origin DNA marks the completion of pre-replication complex formation. If environmental conditions are right in late G1 phase, the G1 and G1/S cyclin- Cdk complexes are activated, which stimulate expression of genes that encode components of the DNA synthetic machinery. G1/S-Cdk activation also promotes the expression and activation of S-Cdk complexes, which may play a role in activating replication origins depending on species and cell type. Control of these Cdks vary depending on cell type and stage of development. This regulation is best understood in budding yeast, where the S cyclins Clb5 and Clb6 are primarily responsible for DNA replication. Clb5,6-Cdk1 complexes directly trigger the activation of replication origins and are therefore required throughout S phase to directly activate each origin. In a similar manner, Cdc7 is also required through S phase to activate replication origins. Cdc7 is not active throughout the cell cycle, and its activation is strictly timed to avoid premature initiation of DNA replication. In late G1, Cdc7 activity rises abruptly as a result of association with the regulatory subunit DBF4, which binds Cdc7 directly and promotes its protein kinase activity. Cdc7 has been found to be a rate-limiting regulator of origin activity. Together, the G1/S-Cdks and/or S-Cdks and Cdc7 collaborate to directly activate the replication origins, leading to initiation of DNA synthesis.

 The replication fork is a structure that forms within the long helical DNA during DNA replication. It is produced by enzymes called helicases that break the hydrogen bonds that hold the DNA strands together in a helix. The resulting structure has two branching "prongs", each one made up of a single strand of DNA. These two strands serve as the template for the leading and lagging strands, which will be created as DNA polymerase matches complementary nucleotides to the templates; the templates may be properly referred to as the leading strand template and the lagging strand template.
DNA is read by DNA polymerase in the 3′ to 5′ direction, meaning the new strand is synthesized in the 5' to 3' direction. Since the leading and lagging strand templates are oriented in opposite directions at the replication fork, a major issue is how to achieve synthesis of new lagging strand DNA, whose direction of synthesis is opposite to the direction of the growing replication fork.
The replication fork is a structure that forms within the long helical DNA during DNA replication. It is produced by enzymes called helicases that break the hydrogen bonds that hold the DNA strands together in a helix. The resulting structure has two branching "prongs", each one made up of a single strand of DNA. These two strands serve as the template for the leading and lagging strands, which will be created as DNA polymerase matches complementary nucleotides to the templates; the templates may be properly referred to as the leading strand template and the lagging strand template.
DNA is read by DNA polymerase in the 3′ to 5′ direction, meaning the new strand is synthesized in the 5' to 3' direction. Since the leading and lagging strand templates are oriented in opposite directions at the replication fork, a major issue is how to achieve synthesis of new lagging strand DNA, whose direction of synthesis is opposite to the direction of the growing replication fork.
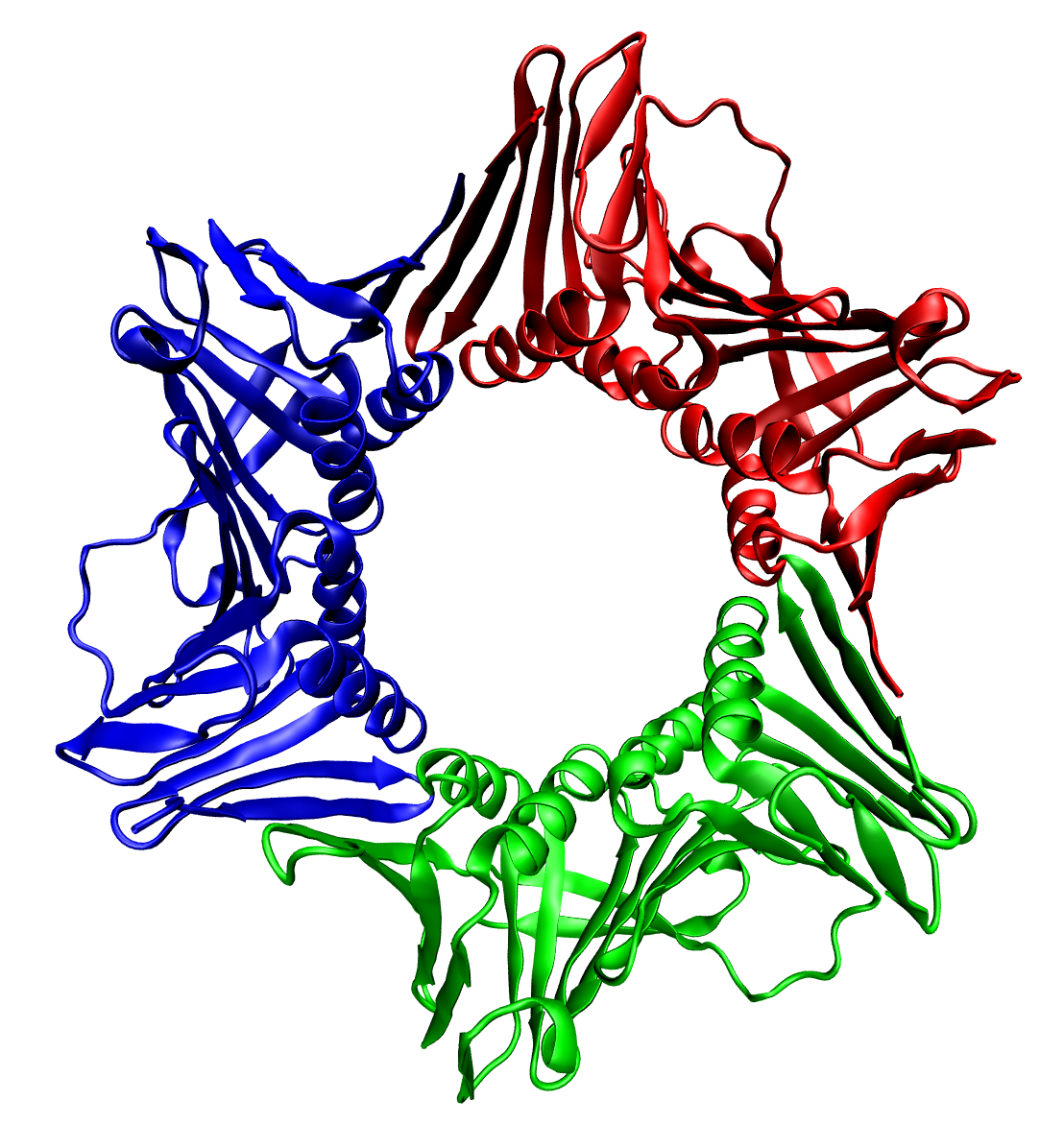 In all cases the helicase is composed of six polypeptides that wrap around only one strand of the DNA being replicated. The two polymerases are bound to the helicase hexamer. In eukaryotes the helicase wraps around the leading strand, and in prokaryotes it wraps around the lagging strand.
As helicase unwinds DNA at the replication fork, the DNA ahead is forced to rotate. This process results in a build-up of twists in the DNA ahead. This build-up creates a torsional load that would eventually stop the replication fork. Topoisomerases are enzymes that temporarily break the strands of DNA, relieving the tension caused by unwinding the two strands of the DNA helix; topoisomerases (including DNA gyrase) achieve this by adding negative supercoils to the DNA helix.
Bare single-stranded DNA tends to fold back on itself forming secondary structures; these structures can interfere with the movement of DNA polymerase. To prevent this, single-strand binding proteins bind to the DNA until a second strand is synthesized, preventing secondary structure formation.
Double-stranded DNA is coiled around histones that play an important role in regulating gene expression so the replicated DNA must be coiled around histones at the same places as the original DNA. To ensure this, histone chaperones disassemble the
In all cases the helicase is composed of six polypeptides that wrap around only one strand of the DNA being replicated. The two polymerases are bound to the helicase hexamer. In eukaryotes the helicase wraps around the leading strand, and in prokaryotes it wraps around the lagging strand.
As helicase unwinds DNA at the replication fork, the DNA ahead is forced to rotate. This process results in a build-up of twists in the DNA ahead. This build-up creates a torsional load that would eventually stop the replication fork. Topoisomerases are enzymes that temporarily break the strands of DNA, relieving the tension caused by unwinding the two strands of the DNA helix; topoisomerases (including DNA gyrase) achieve this by adding negative supercoils to the DNA helix.
Bare single-stranded DNA tends to fold back on itself forming secondary structures; these structures can interfere with the movement of DNA polymerase. To prevent this, single-strand binding proteins bind to the DNA until a second strand is synthesized, preventing secondary structure formation.
Double-stranded DNA is coiled around histones that play an important role in regulating gene expression so the replicated DNA must be coiled around histones at the same places as the original DNA. To ensure this, histone chaperones disassemble the
 Replication machineries consist of factors involved in DNA replication and appearing on template ssDNAs. Replication machineries include primosotors are replication enzymes; DNA polymerase, DNA helicases, DNA clamps and DNA topoisomerases, and replication proteins; e.g. single-stranded DNA binding proteins (SSB). In the replication machineries these components coordinate. In most of the bacteria, all of the factors involved in DNA replication are located on replication forks and the complexes stay on the forks during DNA replication. Replication machineries are also referred to as replisomes, or DNA replication systems. These terms are generic terms for proteins located on replication forks. In eukaryotic and some bacterial cells the replisomes are not formed.
In an alternative figure, DNA factories are similar to projectors and DNAs are like as cinematic films passing constantly into the projectors. In the replication factory model, after both DNA helicases for leading strands and lagging strands are loaded on the template DNAs, the helicases run along the DNAs into each other. The helicases remain associated for the remainder of replication process. Peter Meister et al. observed directly replication sites in budding yeast by monitoring green fluorescent protein (GFP)-tagged DNA polymerases α. They detected DNA replication of pairs of the tagged loci spaced apart symmetrically from a replication origin and found that the distance between the pairs decreased markedly by time. This finding suggests that the mechanism of DNA replication goes with DNA factories. That is, couples of replication factories are loaded on replication origins and the factories associated with each other. Also, template DNAs move into the factories, which bring extrusion of the template ssDNAs and new DNAs. Meister's finding is the first direct evidence of replication factory model. Subsequent research has shown that DNA helicases form dimers in many eukaryotic cells and bacterial replication machineries stay in single intranuclear location during DNA synthesis.
Replication Factories Disentangle Sister Chromatids. The disentanglement is essential for distributing the chromatids into daughter cells after DNA replication. Because sister chromatids after DNA replication hold each other by Cohesin rings, there is the only chance for the disentanglement in DNA replication. Fixing of replication machineries as replication factories can improve the success rate of DNA replication. If replication forks move freely in chromosomes, catenation of nuclei is aggravated and impedes mitotic segregation.
Replication machineries consist of factors involved in DNA replication and appearing on template ssDNAs. Replication machineries include primosotors are replication enzymes; DNA polymerase, DNA helicases, DNA clamps and DNA topoisomerases, and replication proteins; e.g. single-stranded DNA binding proteins (SSB). In the replication machineries these components coordinate. In most of the bacteria, all of the factors involved in DNA replication are located on replication forks and the complexes stay on the forks during DNA replication. Replication machineries are also referred to as replisomes, or DNA replication systems. These terms are generic terms for proteins located on replication forks. In eukaryotic and some bacterial cells the replisomes are not formed.
In an alternative figure, DNA factories are similar to projectors and DNAs are like as cinematic films passing constantly into the projectors. In the replication factory model, after both DNA helicases for leading strands and lagging strands are loaded on the template DNAs, the helicases run along the DNAs into each other. The helicases remain associated for the remainder of replication process. Peter Meister et al. observed directly replication sites in budding yeast by monitoring green fluorescent protein (GFP)-tagged DNA polymerases α. They detected DNA replication of pairs of the tagged loci spaced apart symmetrically from a replication origin and found that the distance between the pairs decreased markedly by time. This finding suggests that the mechanism of DNA replication goes with DNA factories. That is, couples of replication factories are loaded on replication origins and the factories associated with each other. Also, template DNAs move into the factories, which bring extrusion of the template ssDNAs and new DNAs. Meister's finding is the first direct evidence of replication factory model. Subsequent research has shown that DNA helicases form dimers in many eukaryotic cells and bacterial replication machineries stay in single intranuclear location during DNA synthesis.
Replication Factories Disentangle Sister Chromatids. The disentanglement is essential for distributing the chromatids into daughter cells after DNA replication. Because sister chromatids after DNA replication hold each other by Cohesin rings, there is the only chance for the disentanglement in DNA replication. Fixing of replication machineries as replication factories can improve the success rate of DNA replication. If replication forks move freely in chromosomes, catenation of nuclei is aggravated and impedes mitotic segregation.

 Most bacteria do not go through a well-defined cell cycle but instead continuously copy their DNA; during rapid growth, this can result in the concurrent occurrence of multiple rounds of replication. In ''E. coli'', the best-characterized bacteria, DNA replication is regulated through several mechanisms, including: the hemimethylation and sequestering of the origin sequence, the ratio of adenosine triphosphate (ATP) to adenosine diphosphate (ADP), and the levels of protein DnaA. All these control the binding of initiator proteins to the origin sequences.
Because ''E. coli'' methylates GATC DNA sequences, DNA synthesis results in hemimethylated sequences. This hemimethylated DNA is recognized by the protein SeqA, which binds and sequesters the origin sequence; in addition, DnaA (required for initiation of replication) binds less well to hemimethylated DNA. As a result, newly replicated origins are prevented from immediately initiating another round of DNA replication.
ATP builds up when the cell is in a rich medium, triggering DNA replication once the cell has reached a specific size. ATP competes with ADP to bind to DnaA, and the DnaA-ATP complex is able to initiate replication. A certain number of DnaA proteins are also required for DNA replication — each time the origin is copied, the number of binding sites for DnaA doubles, requiring the synthesis of more DnaA to enable another initiation of replication.
In fast-growing bacteria, such as ''E. coli'', chromosome replication takes more time than dividing the cell. The bacteria solve this by initiating a new round of replication before the previous one has been terminated. The new round of replication will form the chromosome of the cell that is born two generations after the dividing cell. This mechanism creates overlapping replication cycles.
Most bacteria do not go through a well-defined cell cycle but instead continuously copy their DNA; during rapid growth, this can result in the concurrent occurrence of multiple rounds of replication. In ''E. coli'', the best-characterized bacteria, DNA replication is regulated through several mechanisms, including: the hemimethylation and sequestering of the origin sequence, the ratio of adenosine triphosphate (ATP) to adenosine diphosphate (ADP), and the levels of protein DnaA. All these control the binding of initiator proteins to the origin sequences.
Because ''E. coli'' methylates GATC DNA sequences, DNA synthesis results in hemimethylated sequences. This hemimethylated DNA is recognized by the protein SeqA, which binds and sequesters the origin sequence; in addition, DnaA (required for initiation of replication) binds less well to hemimethylated DNA. As a result, newly replicated origins are prevented from immediately initiating another round of DNA replication.
ATP builds up when the cell is in a rich medium, triggering DNA replication once the cell has reached a specific size. ATP competes with ADP to bind to DnaA, and the DnaA-ATP complex is able to initiate replication. A certain number of DnaA proteins are also required for DNA replication — each time the origin is copied, the number of binding sites for DnaA doubles, requiring the synthesis of more DnaA to enable another initiation of replication.
In fast-growing bacteria, such as ''E. coli'', chromosome replication takes more time than dividing the cell. The bacteria solve this by initiating a new round of replication before the previous one has been terminated. The new round of replication will form the chromosome of the cell that is born two generations after the dividing cell. This mechanism creates overlapping replication cycles.

 There are many events that contribute to replication stress, including:
* Misincorporation of ribonucleotides
* Unusual DNA structures
* Conflicts between replication and transcription
* Insufficiency of essential replication factors
* Common fragile sites
* Overexpression or constitutive activation of oncogenes
*
There are many events that contribute to replication stress, including:
* Misincorporation of ribonucleotides
* Unusual DNA structures
* Conflicts between replication and transcription
* Insufficiency of essential replication factors
* Common fragile sites
* Overexpression or constitutive activation of oncogenes
*
molecular biology
Molecular biology is a branch of biology that seeks to understand the molecule, molecular basis of biological activity in and between Cell (biology), cells, including biomolecule, biomolecular synthesis, modification, mechanisms, and interactio ...
, DNA replication is the biological process of producing two identical replicas of DNA from one original DNA molecule. DNA replication occurs in all living organisms, acting as the most essential part of biological inheritance. This is essential for cell division during growth and repair of damaged tissues, while it also ensures that each of the new cells receives its own copy of the DNA. The cell possesses the distinctive property of division, which makes replication of DNA essential.
DNA is made up of a double helix
In molecular biology, the term double helix refers to the structure formed by base pair, double-stranded molecules of nucleic acids such as DNA. The double Helix, helical structure of a nucleic acid complex arises as a consequence of its Nuclei ...
of two complementary strands. DNA is often called double helix. The double helix describes the appearance of a double-stranded DNA which is composed of two linear strands that run opposite to each other and twist together. During replication, these strands are separated. Each strand of the original DNA molecule then serves as a template for the production of its counterpart, a process referred to as semiconservative replication. As a result, the new helix will be composed of an original DNA strand as well as a newly synthesized strand. Cellular proofreading and error-checking mechanisms ensure near perfect fidelity
Fidelity is the quality of faithfulness or loyalty. Its original meaning regarded duty in a broader sense than the related concept of '' fealty''. Both derive from the Latin word , meaning "faithful or loyal". In the City of London financial m ...
for DNA replication.Imperfect DNA replication results in mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, ...
s.
In a cell, DNA replication begins at specific locations ( origins of replication) in the genome
A genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding genes, other functional regions of the genome such as ...
which contains the genetic material of an organism. Unwinding of DNA at the origin and synthesis of new strands, accommodated by an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
known as helicase, results in replication forks growing bi-directionally from the origin. A number of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s are associated with the replication fork to help in the initiation and continuation of DNA synthesis. Most prominently, DNA polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create t ...
synthesizes the new strands by adding nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s that complement each (template) strand. DNA replication occurs during the S-stage of interphase.
DNA replication (DNA amplification) can also be performed ''in vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...
'' (artificially, outside a cell). DNA polymerases isolated from cells and artificial DNA primers can be used to start DNA synthesis at known sequences in a template DNA molecule. Polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to make millions to billions of copies of a specific DNA sample rapidly, allowing scientists to amplify a very small sample of DNA (or a part of it) sufficiently to enable detailed st ...
(PCR), ligase chain reaction (LCR), and transcription-mediated amplification
Transcription-mediated amplification (TMA) is an isothermal (performed at constant temperature), single-tube nucleic acid Gene_duplication#As_amplification, amplification system utilizing two enzymes, RNA polymerase and reverse transcriptase.
"Am ...
(TMA) are examples. In March 2021, researchers reported evidence suggesting that a preliminary form of transfer RNA, a necessary component of translation
Translation is the communication of the semantics, meaning of a #Source and target languages, source-language text by means of an Dynamic and formal equivalence, equivalent #Source and target languages, target-language text. The English la ...
, the biological synthesis of new protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s in accordance with the genetic code
Genetic code is a set of rules used by living cell (biology), cells to Translation (biology), translate information encoded within genetic material (DNA or RNA sequences of nucleotide triplets or codons) into proteins. Translation is accomplished ...
, could have been a replicator molecule itself in the very early development of life, or abiogenesis.
DNA structure
DNA is a double-stranded structure, with both strands coiled together to form the characteristicdouble helix
In molecular biology, the term double helix refers to the structure formed by base pair, double-stranded molecules of nucleic acids such as DNA. The double Helix, helical structure of a nucleic acid complex arises as a consequence of its Nuclei ...
. Each single strand of DNA is a chain of four types of nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s. Nucleotides in DNA contain a deoxyribose sugar, a phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
, and a nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
. The four types of nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
correspond to the four nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nuc ...
s adenine, cytosine
Cytosine () (symbol C or Cyt) is one of the four nucleotide bases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attac ...
, guanine
Guanine () (symbol G or Gua) is one of the four main nucleotide bases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside ...
, and thymine, commonly abbreviated as A, C, G, and T. Adenine and guanine are purine bases, while cytosine and thymine are pyrimidines. These nucleotides form phosphodiester bonds, creating the phosphate-deoxyribose backbone of the DNA double helix with the nucleobases pointing inward (i.e., toward the opposing strand). Nucleobases are matched between strands through hydrogen bonds to form base pair
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA ...
s. Adenine pairs with thymine (two hydrogen bonds), and guanine pairs with cytosine (three hydrogen bonds).
DNA strands have a directionality, and the different ends of a single strand are called the "3′ (three-prime) end" and the "5′ (five-prime) end". By convention, if the base sequence of a single strand of DNA is given, the left end of the sequence is the 5′ end, while the right end of the sequence is the 3′ end. The strands of the double helix are anti-parallel, with one being 5′ to 3′, and the opposite strand 3′ to 5′. These terms refer to the carbon atom in deoxyribose to which the next phosphate in the chain attaches. Directionality has consequences in DNA synthesis, because DNA polymerase can synthesize DNA in only one direction by adding nucleotides to the 3′ end of a DNA strand.
The pairing of complementary bases in DNA (through hydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
) means that the information contained within each strand is redundant. Phosphodiester (intra-strand) bonds are stronger than hydrogen (inter-strand) bonds. The actual job of the phosphodiester bonds is where in DNA polymers connect the 5' carbon atom of one nucleotide to the 3' carbon atom of another nucleotide, while the hydrogen bonds stabilize DNA double helices across the helix axis but not in the direction of the axis. This makes it possible to separate the strands from one another. The nucleotides on a single strand can therefore be used to reconstruct nucleotides on a newly synthesized partner strand.
DNA polymerase
DNA polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create t ...
s are a family of enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s that carry out all forms of DNA replication. DNA polymerases in general cannot initiate synthesis of new strands but can only extend an existing DNA or RNA strand paired with a template strand. To begin synthesis, a short fragment of RNA, called a primer, must be created and paired with the template DNA strand.
DNA polymerase adds a new strand of DNA by extending the 3′ end of an existing nucleotide chain, adding new nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s matched to the template strand, one at a time, via the creation of phosphodiester bond
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is d ...
s. The energy for this process of DNA polymerization comes from hydrolysis of the high-energy phosphate (phosphoanhydride) bonds between the three phosphates attached to each unincorporated base. Free bases with their attached phosphate groups are called nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s; in particular, bases with three attached phosphate groups are called nucleoside triphosphates. When a nucleotide is being added to a growing DNA strand, the formation of a phosphodiester bond between the proximal phosphate of the nucleotide to the growing chain is accompanied by hydrolysis of a high-energy phosphate bond with release of the two distal phosphate groups as a pyrophosphate. Enzymatic hydrolysis of the resulting pyrophosphate into inorganic phosphate consumes a second high-energy phosphate bond and renders the reaction effectively irreversible.The energetics of this process may also help explain the directionality of synthesis—if DNA were synthesized in the 3′ to 5′ direction, the energy for the process would come from the 5′ end of the growing strand rather than from free nucleotides. The problem is that if the high energy triphosphates were on the growing strand and not on the free nucleotides, proof-reading by removing a mismatched terminal nucleotide would be problematic: Once a nucleotide is added, the triphosphate is lost and a single phosphate remains on the backbone between the new nucleotide and the rest of the strand. If the added nucleotide were mismatched, removal would result in a DNA strand terminated by a monophosphate at the end of the "growing strand" rather than a high energy triphosphate. So strand would be stuck and wouldn't be able to grow anymore. In actuality, the high energy triphosphates hydrolyzed at each step originate from the free nucleotides, not the polymerized strand, so this issue does not exist.
In general, DNA polymerases are highly accurate, with an intrinsic error rate of less than one mistake for every 107 nucleotides added. Some DNA polymerases can also delete nucleotides from the end of a developing strand in order to fix mismatched bases. This is known as proofreading. Finally, post-replication mismatch repair mechanisms monitor the DNA for errors, being capable of distinguishing mismatches in the newly synthesized DNA Strand from the original strand sequence. Together, these three discrimination steps enable replication fidelity of less than one mistake for every 109 nucleotides added.
The rate of DNA replication in a living cell was first measured as the rate of phage T4 DNA elongation in phage-infected ''E. coli''. During the period of exponential DNA increase at 37 °C, the rate was 749 nucleotides per second. The mutation rate per base pair per replication during phase T4 DNA synthesis is 1.7 per 108.
Replication process
Initiation

 For a cell to divide, it must first replicate its DNA. DNA replication is an all-or-none process; once replication begins, it proceeds to completion. Once replication is complete, it does not occur again in the same cell cycle. This is made possible by the division of initiation of th
For a cell to divide, it must first replicate its DNA. DNA replication is an all-or-none process; once replication begins, it proceeds to completion. Once replication is complete, it does not occur again in the same cell cycle. This is made possible by the division of initiation of thpre-replication complex
Pre-replication complex
In latemitosis
Mitosis () is a part of the cell cycle in eukaryote, eukaryotic cells in which replicated chromosomes are separated into two new Cell nucleus, nuclei. Cell division by mitosis is an equational division which gives rise to genetically identic ...
and early G1 phase
The G1 phase, gap 1 phase, or growth 1 phase, is the first of four phases of the cell cycle that takes place in eukaryotic cell division. In this part of interphase, the cell synthesizes Messenger RNA, mRNA and proteins in preparation for subsequ ...
, a large complex of initiator proteins assembles into the pre-replication complex at particular points in the DNA, known as " origins". In '' E. coli'' the primary initiator protein is Dna A; in yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are est ...
, this is the origin recognition complex. Sequences used by initiator proteins tend to be "AT-rich" (rich in adenine and thymine bases), because A-T base pairs have two hydrogen bonds (rather than the three formed in a C-G pair) and thus are easier to strand-separate. In eukaryotes, the origin recognition complex catalyzes the assembly of initiator proteins into the pre-replication complex. In addition, a recent report suggests that budding yeast ORC dimerizes in a cell cycle dependent manner to control licensing. In turn, the process of ORC dimerization is mediated by a cell cycle-dependent Noc3p dimerization cycle in vivo, and this role of Noc3p is separable from its role in ribosome biogenesisAn essential Noc3p dimerization cycle mediates ORC double-hexamer formation in replication licensing
ORC and Noc3p are continuously bound to the chromatin throughout the cell cycle. Cdc6 and Cdt1 then associate with the bound origin recognition complex at the origin in order to form a larger complex necessary to load the Mcm complex onto the DNA. In eukaryotes, the Mcm complex is the helicase that will split the DNA helix at the replication forks and origins. The Mcm complex is recruited at late G1 phase and loaded by the ORC-Cdc6-Cdt1 complex onto the DNA via ATP-dependent protein remodeling. The loading of the MCM complex onto the origin DNA marks the completion of pre-replication complex formation. If environmental conditions are right in late G1 phase, the G1 and G1/S cyclin- Cdk complexes are activated, which stimulate expression of genes that encode components of the DNA synthetic machinery. G1/S-Cdk activation also promotes the expression and activation of S-Cdk complexes, which may play a role in activating replication origins depending on species and cell type. Control of these Cdks vary depending on cell type and stage of development. This regulation is best understood in budding yeast, where the S cyclins Clb5 and Clb6 are primarily responsible for DNA replication. Clb5,6-Cdk1 complexes directly trigger the activation of replication origins and are therefore required throughout S phase to directly activate each origin. In a similar manner, Cdc7 is also required through S phase to activate replication origins. Cdc7 is not active throughout the cell cycle, and its activation is strictly timed to avoid premature initiation of DNA replication. In late G1, Cdc7 activity rises abruptly as a result of association with the regulatory subunit DBF4, which binds Cdc7 directly and promotes its protein kinase activity. Cdc7 has been found to be a rate-limiting regulator of origin activity. Together, the G1/S-Cdks and/or S-Cdks and Cdc7 collaborate to directly activate the replication origins, leading to initiation of DNA synthesis.
Preinitiation complex
In early S phase, S-Cdk and Cdc7 activation lead to the assembly of the preinitiation complex, a massive protein complex formed at the origin. Formation of the preinitiation complex displaces Cdc6 and Cdt1 from the origin replication complex, inactivating and disassembling the pre-replication complex. Loading the preinitiation complex onto the origin activates the Mcm helicase, causing unwinding of the DNA helix. The preinitiation complex also loads α-primase and other DNA polymerases onto the DNA. After α-primase synthesizes the first primers, the primer-template junctions interact with the clamp loader, which loads the sliding clamp onto the DNA to begin DNA synthesis. The components of the preinitiation complex remain associated with replication forks as they move out from the origin.Elongation
DNA polymerase has 5′–3′ activity. All known DNA replication systems require a free 3′ hydroxyl group before synthesis can be initiated (note: the DNA template is read in 3′ to 5′ direction whereas a new strand is synthesized in the 5′ to 3′ direction—this is often confused). Four distinct mechanisms for DNA synthesis are recognized: # All cellular life forms and many DNAvirus
A virus is a submicroscopic infectious agent that replicates only inside the living Cell (biology), cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses are ...
es, phages and plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria and ...
s use a primase to synthesize a short RNA primer with a free 3′ OH group which is subsequently elongated by a DNA polymerase.
# The retroelements (including retroviruses) employ a transfer RNA that primes DNA replication by providing a free 3′ OH that is used for elongation by the reverse transcriptase.
# In the adenoviruses and the φ29 family of bacteriophage
A bacteriophage (), also known informally as a phage (), is a virus that infects and replicates within bacteria. The term is derived . Bacteriophages are composed of proteins that Capsid, encapsulate a DNA or RNA genome, and may have structu ...
s, the 3′ OH group is provided by the side chain of an amino acid of the genome attached protein (the terminal protein) to which nucleotides are added by the DNA polymerase to form a new strand.
# In the single stranded DNA viruses—a group that includes the circovirus
''Circovirus'' is a genus of viruses, in the family ''Circoviridae''. Birds (such as pigeons and ducks) and pigs serve as natural hosts, though dogs have been shown to be infected as well. Circoviruses are single stranded DNA (ssDNA) viruses. The ...
es, the geminiviruses, the parvoviruses and others—and also the many phages and plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria and ...
s that use the rolling circle replication (RCR) mechanism, the RCR endonuclease creates a nick in the genome strand (single stranded viruses) or one of the DNA strands (plasmids). The 5′ end of the nicked strand is transferred to a tyrosine residue on the nuclease and the free 3′ OH group is then used by the DNA polymerase to synthesize the new strand.
Cellular organisms use the first of these pathways since it is the most well-known. In this mechanism, once the two strands are separated, primase adds RNA primers to the template strands. The leading strand receives one RNA primer while the lagging strand receives several. The leading strand is continuously extended from the primer by a DNA polymerase with high processivity, while the lagging strand is extended discontinuously from each primer forming Okazaki fragments
Okazaki fragments are short sequences of DNA nucleotides (approximately 150 to 200 base pairs long in eukaryotes) which are synthesized discontinuously and later linked together by the enzyme DNA ligase to create the lagging strand during DN ...
. RNase removes the primer RNA fragments, and a low processivity DNA polymerase distinct from the replicative polymerase enters to fill the gaps. When this is complete, a single nick on the leading strand and several nicks on the lagging strand can be found. Ligase works to fill these nicks in, thus completing the newly replicated DNA molecule.
The primase used in this process differs significantly between bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
and archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
/eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s. Bacteria use a primase belonging to the DnaG protein superfamily which contains a catalytic domain of the TOPRIM fold type. The TOPRIM fold contains an α/β core with four conserved strands in a Rossmann-like topology. This structure is also found in the catalytic domains of topoisomerase Ia, topoisomerase II, the OLD-family nucleases and DNA repair proteins related to the RecR protein.
The primase used by archaea and eukaryotes, in contrast, contains a highly derived version of the RNA recognition motif (RRM). This primase is structurally similar to many viral RNA-dependent RNA polymerases, reverse transcriptases, cyclic nucleotide generating cyclases and DNA polymerases of the A/B/Y families that are involved in DNA replication and repair. In eukaryotic replication, the primase forms a complex with Pol α.
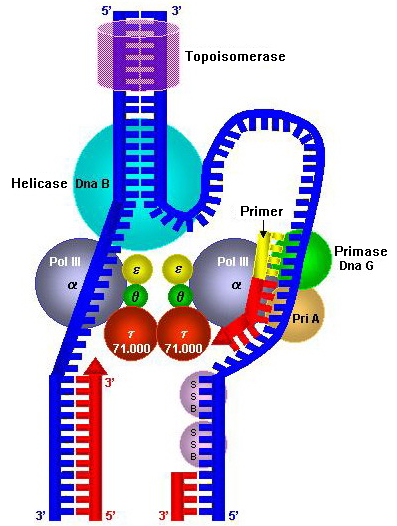
Multiple DNA polymerases take on different roles in the DNA replication process. In '' E. coli'', DNA Pol III is the polymerase enzyme primarily responsible for DNA replication. It assembles into a replication complex at the replication fork that exhibits extremely high processivity, remaining intact for the entire replication cycle. In contrast, DNA Pol I is the enzyme responsible for replacing RNA primers with DNA. DNA Pol I has a 5′ to 3′ exonuclease activity in addition to its polymerase activity, and uses its exonuclease activity to degrade the RNA primers ahead of it as it extends the DNA strand behind it, in a process called nick translation. Pol I is much less processive than Pol III because its primary function in DNA replication is to create many short DNA regions rather than a few very long regions.
In eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s, the low-processivity enzyme, Pol α, helps to initiate replication because it forms a complex with primase. In eukaryotes, leading strand synthesis is thought to be conducted by Pol ε; however, this view has recently been challenged, suggesting a role for Pol δ. Primer removal is completed Pol δ while repair of DNA during replication is completed by Pol ε.
As DNA synthesis continues, the original DNA strands continue to unwind on each side of the bubble, forming a replication fork with two prongs. In bacteria, which have a single origin of replication on their circular chromosome, this process creates a " theta structure" (resembling the Greek letter theta: θ). In contrast, eukaryotes have longer linear chromosomes and initiate replication at multiple origins within these.
Replication fork
Leading strand
The leading strand is the strand of new DNA which is synthesized in the same direction as the growing replication fork. This sort of DNA replication is continuous.Lagging strand
The lagging strand is the strand of new DNA whose direction of synthesis is opposite to the direction of the growing replication fork. Because of its orientation, replication of the lagging strand is more complicated as compared to that of the leading strand. As a consequence, the DNA polymerase on this strand is seen to "lag behind" the other strand. The lagging strand is synthesized in short, separated segments. On the lagging strand ''template'', a primase "reads" the template DNA and initiates synthesis of a short complementaryRNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
primer. A DNA polymerase extends the primed segments, forming Okazaki fragments. The RNA primers are then removed and replaced with DNA, and the fragments of DNA are joined by DNA ligase.
Dynamics at the replication fork
 In all cases the helicase is composed of six polypeptides that wrap around only one strand of the DNA being replicated. The two polymerases are bound to the helicase hexamer. In eukaryotes the helicase wraps around the leading strand, and in prokaryotes it wraps around the lagging strand.
As helicase unwinds DNA at the replication fork, the DNA ahead is forced to rotate. This process results in a build-up of twists in the DNA ahead. This build-up creates a torsional load that would eventually stop the replication fork. Topoisomerases are enzymes that temporarily break the strands of DNA, relieving the tension caused by unwinding the two strands of the DNA helix; topoisomerases (including DNA gyrase) achieve this by adding negative supercoils to the DNA helix.
Bare single-stranded DNA tends to fold back on itself forming secondary structures; these structures can interfere with the movement of DNA polymerase. To prevent this, single-strand binding proteins bind to the DNA until a second strand is synthesized, preventing secondary structure formation.
Double-stranded DNA is coiled around histones that play an important role in regulating gene expression so the replicated DNA must be coiled around histones at the same places as the original DNA. To ensure this, histone chaperones disassemble the
In all cases the helicase is composed of six polypeptides that wrap around only one strand of the DNA being replicated. The two polymerases are bound to the helicase hexamer. In eukaryotes the helicase wraps around the leading strand, and in prokaryotes it wraps around the lagging strand.
As helicase unwinds DNA at the replication fork, the DNA ahead is forced to rotate. This process results in a build-up of twists in the DNA ahead. This build-up creates a torsional load that would eventually stop the replication fork. Topoisomerases are enzymes that temporarily break the strands of DNA, relieving the tension caused by unwinding the two strands of the DNA helix; topoisomerases (including DNA gyrase) achieve this by adding negative supercoils to the DNA helix.
Bare single-stranded DNA tends to fold back on itself forming secondary structures; these structures can interfere with the movement of DNA polymerase. To prevent this, single-strand binding proteins bind to the DNA until a second strand is synthesized, preventing secondary structure formation.
Double-stranded DNA is coiled around histones that play an important role in regulating gene expression so the replicated DNA must be coiled around histones at the same places as the original DNA. To ensure this, histone chaperones disassemble the chromatin
Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important r ...
before it is replicated and replace the histones in the correct place. Some steps in this reassembly are somewhat speculative.
Clamp proteins act as a sliding clamp on DNA, allowing the DNA polymerase to bind to its template and aid in processivity. The inner face of the clamp enables DNA to be threaded through it. Once the polymerase reaches the end of the template or detects double-stranded DNA, the sliding clamp undergoes a conformational change that releases the DNA polymerase. Clamp-loading proteins are used to initially load the clamp, recognizing the junction between template and RNA primers.:274-5
DNA replication proteins
At the replication fork, many replication enzymes assemble on the DNA into a complex molecular machine called the replisome. The following is a list of major DNA replication enzymes that participate in the replisome: ''In vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in ...
'' single-molecule experiments (using optical tweezers and magnetic tweezers) have found synergetic interactions between the replisome enzymes ( helicase, polymerase
In biochemistry, a polymerase is an enzyme (Enzyme Commission number, EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by ...
, and Single-strand DNA-binding protein) and with the DNA replication fork enhancing DNA-unwinding and DNA-replication. These results lead to the development of kinetic models accounting for the synergetic interactions and their stability.
Replication machinery
 Replication machineries consist of factors involved in DNA replication and appearing on template ssDNAs. Replication machineries include primosotors are replication enzymes; DNA polymerase, DNA helicases, DNA clamps and DNA topoisomerases, and replication proteins; e.g. single-stranded DNA binding proteins (SSB). In the replication machineries these components coordinate. In most of the bacteria, all of the factors involved in DNA replication are located on replication forks and the complexes stay on the forks during DNA replication. Replication machineries are also referred to as replisomes, or DNA replication systems. These terms are generic terms for proteins located on replication forks. In eukaryotic and some bacterial cells the replisomes are not formed.
In an alternative figure, DNA factories are similar to projectors and DNAs are like as cinematic films passing constantly into the projectors. In the replication factory model, after both DNA helicases for leading strands and lagging strands are loaded on the template DNAs, the helicases run along the DNAs into each other. The helicases remain associated for the remainder of replication process. Peter Meister et al. observed directly replication sites in budding yeast by monitoring green fluorescent protein (GFP)-tagged DNA polymerases α. They detected DNA replication of pairs of the tagged loci spaced apart symmetrically from a replication origin and found that the distance between the pairs decreased markedly by time. This finding suggests that the mechanism of DNA replication goes with DNA factories. That is, couples of replication factories are loaded on replication origins and the factories associated with each other. Also, template DNAs move into the factories, which bring extrusion of the template ssDNAs and new DNAs. Meister's finding is the first direct evidence of replication factory model. Subsequent research has shown that DNA helicases form dimers in many eukaryotic cells and bacterial replication machineries stay in single intranuclear location during DNA synthesis.
Replication Factories Disentangle Sister Chromatids. The disentanglement is essential for distributing the chromatids into daughter cells after DNA replication. Because sister chromatids after DNA replication hold each other by Cohesin rings, there is the only chance for the disentanglement in DNA replication. Fixing of replication machineries as replication factories can improve the success rate of DNA replication. If replication forks move freely in chromosomes, catenation of nuclei is aggravated and impedes mitotic segregation.
Replication machineries consist of factors involved in DNA replication and appearing on template ssDNAs. Replication machineries include primosotors are replication enzymes; DNA polymerase, DNA helicases, DNA clamps and DNA topoisomerases, and replication proteins; e.g. single-stranded DNA binding proteins (SSB). In the replication machineries these components coordinate. In most of the bacteria, all of the factors involved in DNA replication are located on replication forks and the complexes stay on the forks during DNA replication. Replication machineries are also referred to as replisomes, or DNA replication systems. These terms are generic terms for proteins located on replication forks. In eukaryotic and some bacterial cells the replisomes are not formed.
In an alternative figure, DNA factories are similar to projectors and DNAs are like as cinematic films passing constantly into the projectors. In the replication factory model, after both DNA helicases for leading strands and lagging strands are loaded on the template DNAs, the helicases run along the DNAs into each other. The helicases remain associated for the remainder of replication process. Peter Meister et al. observed directly replication sites in budding yeast by monitoring green fluorescent protein (GFP)-tagged DNA polymerases α. They detected DNA replication of pairs of the tagged loci spaced apart symmetrically from a replication origin and found that the distance between the pairs decreased markedly by time. This finding suggests that the mechanism of DNA replication goes with DNA factories. That is, couples of replication factories are loaded on replication origins and the factories associated with each other. Also, template DNAs move into the factories, which bring extrusion of the template ssDNAs and new DNAs. Meister's finding is the first direct evidence of replication factory model. Subsequent research has shown that DNA helicases form dimers in many eukaryotic cells and bacterial replication machineries stay in single intranuclear location during DNA synthesis.
Replication Factories Disentangle Sister Chromatids. The disentanglement is essential for distributing the chromatids into daughter cells after DNA replication. Because sister chromatids after DNA replication hold each other by Cohesin rings, there is the only chance for the disentanglement in DNA replication. Fixing of replication machineries as replication factories can improve the success rate of DNA replication. If replication forks move freely in chromosomes, catenation of nuclei is aggravated and impedes mitotic segregation.
Termination
Eukaryotes initiate DNA replication at multiple points in the chromosome, so replication forks meet and terminate at many points in the chromosome. Because eukaryotes have linear chromosomes, DNA replication is unable to reach the very end of the chromosomes. Due to this problem, DNA is lost in each replication cycle from the end of the chromosome. Telomeres are regions of repetitive DNA close to the ends and help prevent loss of genes due to this shortening. Shortening of the telomeres is a normal process in somatic cells. This shortens the telomeres of the daughter DNA chromosome. As a result, cells can only divide a certain number of times before the DNA loss prevents further division. (This is known as the Hayflick limit.) Within thegerm cell
A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they unde ...
line, which passes DNA to the next generation, telomerase extends the repetitive sequences of the telomere region to prevent degradation. Telomerase can become mistakenly active in somatic cells, sometimes leading to cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
formation. Increased telomerase activity is one of the hallmarks of cancer.
Termination requires that the progress of the DNA replication fork must stop or be blocked. Termination at a specific locus, when it occurs, involves the interaction between two components: (1) a termination site sequence in the DNA, and (2) a protein which binds to this sequence to physically stop DNA replication. In various bacterial species, this is named the DNA replication terminus site-binding protein, or Ter protein.
Because bacteria have circular chromosomes, termination of replication occurs when the two replication forks meet each other on the opposite end of the parental chromosome. ''E. coli'' regulates this process through the use of termination sequences that, when bound by the Tus protein, enable only one direction of replication fork to pass through. As a result, the replication forks are constrained to always meet within the termination region of the chromosome.
Regulation
Eukaryotes
Within eukaryotes, DNA replication is controlled within the context of thecell cycle
The cell cycle, or cell-division cycle, is the sequential series of events that take place in a cell (biology), cell that causes it to divide into two daughter cells. These events include the growth of the cell, duplication of its DNA (DNA re ...
. As the cell grows and divides, it progresses through stages in the cell cycle; DNA replication takes place during the S phase (synthesis phase). The progress of the eukaryotic cell through the cycle is controlled by cell cycle checkpoints. Progression through checkpoints is controlled through complex interactions between various proteins, including cyclins and cyclin-dependent kinase
Cyclin-dependent kinases (CDKs) are a predominant group of serine/threonine protein kinases involved in the regulation of the cell cycle and its progression, ensuring the integrity and functionality of cellular machinery. These regulatory enzym ...
s. Unlike bacteria, eukaryotic DNA replicates in the confines of the nucleus.
The G1/S checkpoint (restriction checkpoint) regulates whether eukaryotic cells enter the process of DNA replication and subsequent division. Cells that do not proceed through this checkpoint remain in the G0 stage and do not replicate their DNA.
Once the DNA has gone through the "G1/S" test, it can only be copied once in every cell cycle. When the Mcm complex moves away from the origin, the pre-replication complex is dismantled. Because a new Mcm complex cannot be loaded at an origin until the pre-replication subunits are reactivated, one origin of replication can not be used twice in the same cell cycle.
Activation of S-Cdks in early S phase promotes the destruction or inhibition of individual pre-replication complex components, preventing immediate reassembly. S and M-Cdks continue to block pre-replication complex assembly even after S phase is complete, ensuring that assembly cannot occur again until all Cdk activity is reduced in late mitosis.
In budding yeast, inhibition of assembly is caused by Cdk-dependent phosphorylation of pre-replication complex components. At the onset of S phase, phosphorylation of Cdc6 by Cdk1 causes the binding of Cdc6 to the SCF ubiquitin protein ligase, which causes proteolytic destruction of Cdc6. Cdk-dependent phosphorylation of Mcm proteins promotes their export out of the nucleus along with Cdt1 during S phase, preventing the loading of new Mcm complexes at origins during a single cell cycle. Cdk phosphorylation of the origin replication complex also inhibits pre-replication complex assembly. The individual presence of any of these three mechanisms is sufficient to inhibit pre-replication complex assembly. However, mutations of all three proteins in the same cell does trigger reinitiation at many origins of replication within one cell cycle.
In animal cells, the protein geminin is a key inhibitor of pre-replication complex assembly. Geminin binds Cdt1, preventing its binding to the origin recognition complex. In G1, levels of geminin are kept low by the APC, which ubiquitinates geminin to target it for degradation. When geminin is destroyed, Cdt1 is released, allowing it to function in pre-replication complex assembly. At the end of G1, the APC is inactivated, allowing geminin to accumulate and bind Cdt1.
Replication of chloroplast and mitochondrial genomes occurs independently of the cell cycle, through the process of D-loop replication.
Replication focus
In vertebrate cells, replication sites concentrate into positions called replication foci. Replication sites can be detected by immunostaining daughter strands and replication enzymes and monitoring GFP-tagged replication factors. By these methods it is found that replication foci of varying size and positions appear in S phase of cell division and their number per nucleus is far smaller than the number of genomic replication forks. P. Heun et al.,(2001) tracked GFP-tagged replication foci in budding yeast cells and revealed that replication origins move constantly in G1 and S phase and the dynamics decreased significantly in S phase. Traditionally, replication sites were fixed on spatial structure of chromosomes by nuclear matrix or lamins. The Heun's results denied the traditional concepts, budding yeasts do not have lamins, and support that replication origins self-assemble and form replication foci. By firing of replication origins, controlled spatially and temporally, the formation of replication foci is regulated. D. A. Jackson et al.(1998) revealed that neighboring origins fire simultaneously in mammalian cells. Spatial juxtaposition of replication sites brings clustering of replication forks. The clustering do rescue of stalled replication forks and favors normal progress of replication forks. Progress of replication forks is inhibited by many factors; collision with proteins or with complexes binding strongly on DNA, deficiency of dNTPs, nicks on template DNAs and so on. If replication forks get stuck and the rest of the sequences from the stuck forks are not copied, then the daughter strands get nick nick unreplicated sites. The un-replicated sites on one parent's strand hold the other strand together but not daughter strands. Therefore, the resulting sister chromatids cannot separate from each other and cannot divide into 2 daughter cells. When neighboring origins fire and a fork from one origin is stalled, fork from other origin access on an opposite direction of the stalled fork and duplicate the un-replicated sites. As other mechanism of the rescue there is application of dormant replication origins that excess origins do not fire in normal DNA replication.Bacteria
Problems with DNA replication

 There are many events that contribute to replication stress, including:
* Misincorporation of ribonucleotides
* Unusual DNA structures
* Conflicts between replication and transcription
* Insufficiency of essential replication factors
* Common fragile sites
* Overexpression or constitutive activation of oncogenes
*
There are many events that contribute to replication stress, including:
* Misincorporation of ribonucleotides
* Unusual DNA structures
* Conflicts between replication and transcription
* Insufficiency of essential replication factors
* Common fragile sites
* Overexpression or constitutive activation of oncogenes
* Chromatin
Chromatin is a complex of DNA and protein found in eukaryote, eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important r ...
inaccessibility
Polymerase chain reaction
Researchers commonly replicate DNA ''in vitro'' using thepolymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to make millions to billions of copies of a specific DNA sample rapidly, allowing scientists to amplify a very small sample of DNA (or a part of it) sufficiently to enable detailed st ...
(PCR). PCR uses a pair of primers to span a target region in template DNA, and then polymerizes partner strands in each direction from these primers using a thermostable DNA polymerase. Repeating this process through multiple cycles amplifies the targeted DNA region. At the start of each cycle, the mixture of template and primers is heated, separating the newly synthesized molecule and template. Then, as the mixture cools, both of these become templates for annealing of new primers, and the polymerase extends from these. As a result, the number of copies of the target region doubles each round, increasing exponentially.
See also
* Autopoiesis *Cell (biology)
The cell is the basic structural and functional unit of all life, forms of life. Every cell consists of cytoplasm enclosed within a Cell membrane, membrane; many cells contain organelles, each with a specific function. The term comes from the ...
* Cell division
* Chromosome segregation
* Data storage device
* Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
* Gene expression
Gene expression is the process (including its Regulation of gene expression, regulation) by which information from a gene is used in the synthesis of a functional gene product that enables it to produce end products, proteins or non-coding RNA, ...
* Epigenetics
In biology, epigenetics is the study of changes in gene expression that happen without changes to the DNA sequence. The Greek prefix ''epi-'' (ἐπι- "over, outside of, around") in ''epigenetics'' implies features that are "on top of" or "in ...
* Genome
A genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding genes, other functional regions of the genome such as ...
* Hachimoji DNA
* Life
Life, also known as biota, refers to matter that has biological processes, such as Cell signaling, signaling and self-sustaining processes. It is defined descriptively by the capacity for homeostasis, Structure#Biological, organisation, met ...
* Replication (computing)
* Self-replication
Notes
References
{{Authority control DNA replication Senescence Cellular processes Molecular biology Copying