Cystathionine gamma-lyase on:
[Wikipedia]
[Google]
[Amazon]
The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into

 Cysteine is the rate-limiting substrate in the synthetic pathway for
Cysteine is the rate-limiting substrate in the synthetic pathway for 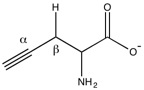 Excessive levels of H2S, due to increased activity of cystathionase, are associated with endotoxemia,
Excessive levels of H2S, due to increased activity of cystathionase, are associated with endotoxemia,
cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
, 2-oxobutanoate ( α-ketobutyrate), and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
:
:L-cystathionine + H2O = L-cysteine + 2-oxobutanoate + NH3 (overall reaction)
::(1a) L-cystathionine = L-cysteine + 2-aminobut-2-enoate
::(1b) 2-aminobut-2-enoate = 2-iminobutanoate (spontaneous)
::(1c) 2-iminobutanoate + H2O = 2-oxobutanoate + NH3 (spontaneous)
Pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'- phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent ...
is a prosthetic group
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein.
Not to be confused with the cofactor that binds to the enzyme apoenzyme (eith ...
of this enzyme.
Cystathionine γ-lyase also catalyses the following elimination reactions:
* L- homoserine to form H2O, NH3 and 2-oxobutanoate
* L-cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mec ...
, producing thiocysteine, pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic a ...
and NH3
* L-cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, some ...
producing pyruvate, NH3 and H2S
In some bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
and mammal
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur ...
s, including humans, this enzyme takes part in generating hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
. Hydrogen sulfide is one of a few gases that was recently discovered to have a role in cell signaling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellula ...
in the body.
Enzyme mechanism
Cystathionase uses pyridoxal phosphate to facilitate the cleavage of the sulfur-gamma carbon bond of cystathionine, resulting in the release of cysteine. Thelysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated − ...
residue reforms the internal aldimine by kicking off α-iminobutyric acid. Afterwards the external ketimine is hydrolyzed, causing the formation of α-ketobutyrate.
The amino group on cystathionine is deprotonated and undergoes a nucleophilic attack of the internal aldimine. An additional deprotonation by a general base results in the formation of the external aldimine and removal of the lysine residue. The basic lysine residue is then able to deprotonate the alpha carbon, pushing electron density into the nitrogen of the pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
ring. Pyridoxal phosphate is necessary to stabilize this carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3 ...
ic intermediate; otherwise the proton's pKa
PKA may refer to:
* Professionally known as:
** Pen name
** Stage persona
* p''K''a, the symbol for the acid dissociation constant at logarithmic scale
* Protein kinase A, a class of cAMP-dependent enzymes
* Pi Kappa Alpha, the North-American so ...
would be too high. The beta carbon is then deprotonated, creating an alpha-beta unsaturation and pushing a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. L ...
onto the aldimine nitrogen. To reform the aldimine, this lone pair pushes back down, cleaving the sulfur-gamma carbon bond, resulting in the release of cysteine.
A pyridoxamine derivative of vinyl glyoxylate
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Str ...
remains after the gamma elimination. The lone pair from the pyridine nitrogen pushes electron density to the gamma carbon, which is protonated by lysine. Lysine then attacks the external aldimine, pushing electron density to the beta carbon, which is protonated by a general acid. The imine is then hydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
to release α-ketobutyrate. Deprotonation of the lysine residue causes ammonia to leave, thus completing the catalytic cycle.
Cystathionine gamma lyase also shows gamma-synthase activity depending on the concentrations of reactants present. The mechanisms are the same until they diverge after formation of the vinyl glyoxylate derivative. In the gamma synthase mechanism, the gamma carbon is attacked by a sulfur nucleophile, resulting in the formation of a new sulfur-gamma carbon bond.

Enzyme structure
Cystathionine γ-lyase is a member of the Cys/Met metabolism PLP-dependent enzymes family. Other members include cystathionine γ synthase, cystathionine β lyase, and methionine γ lyase. It is also a member of the broader aspartate aminotransferase family. Like many other PLP-dependent enzymes, cystathionine γ-lyase is atetramer
A tetramer () ('' tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
with D2 symmetry.
Pyridoxal phosphate is bound in the active site by Lys212.
Disease relevance
 Cysteine is the rate-limiting substrate in the synthetic pathway for
Cysteine is the rate-limiting substrate in the synthetic pathway for glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
in the eye. Glutathione is an antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubrica ...
that protects crystallins in the eye from reactive oxygen species; denatured crystallins can lead to cataracts
A cataract is a cloudy area in the lens of the eye that leads to a decrease in vision. Cataracts often develop slowly and can affect one or both eyes. Symptoms may include faded colors, blurry or double vision, halos around light, trouble ...
. Cystathionase is also a target for reactive oxygen species. Thus as cystathionase is oxidized, its activity decreases, causing a decrease in cysteine and, in turn, glutathione in the eye, leading to a decrease in antioxidant availability, causing a further decrease in cystathionase activity. Deficiencies in cystathionase activity have also been shown to contribute to glutathione depletion in patients with cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
and AIDS
Human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS) is a spectrum of conditions caused by infection with the human immunodeficiency virus (HIV), a retrovirus. Following initial infection an individual ma ...
.
Mutations
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, m ...
and deficiencies in cystathionase are associated with cystathioninuria. The mutations T67I and Q240E weaken the enzyme's affinity for pyridoxal phosphate, the co-factor vital to enzymatic function. Low levels of H2S have also been associated with hypertension
Hypertension (HTN or HT), also known as high blood pressure (HBP), is a long-term medical condition in which the blood pressure in the arteries is persistently elevated. High blood pressure usually does not cause symptoms. Long-term high b ...
in mice. Excessive levels of H2S, due to increased activity of cystathionase, are associated with endotoxemia,
Excessive levels of H2S, due to increased activity of cystathionase, are associated with endotoxemia, acute pancreatitis
Acute pancreatitis (AP) is a sudden inflammation of the pancreas. Causes in order of frequency include: 1) a gallstone impacted in the common bile duct beyond the point where the pancreatic duct joins it; 2) heavy alcohol use; 3) systemic disea ...
, hemorrhagic shock, and diabetes mellitus
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ...
.
Propargylglycine and β-cyanoalanine are two irreversible inhibitors of cystathionase used to treat elevated H2S levels. Mechanistically, the amino group of propargylglycine attacks the aldimine to form an external aldimine. The β position of the alkyne is then deprotonated to form the allene, which is then attacked by the phenol of Tyr114. The internal aldimine can regenerate, but the newly created vinyl ether sterically hinders the active site, blocking cysteine from attacking pyridoxal phosphate.
Regulation
H2S decreases transcription of cystathionase at concentrations between 10 and 80μM. However, transcription is increased by concentrations near 120μM, and inhibited completely at concentrations in excess of 160μM.See also
* Cysteine metabolismReferences
External links
* {{Portal bar, Biology, border=no EC 4.4.1