Cyclopentadienyliron dicarbonyl dimer on:
[Wikipedia]
[Google]
[Amazon]
Cyclopentadienyliron dicarbonyl dimer is an
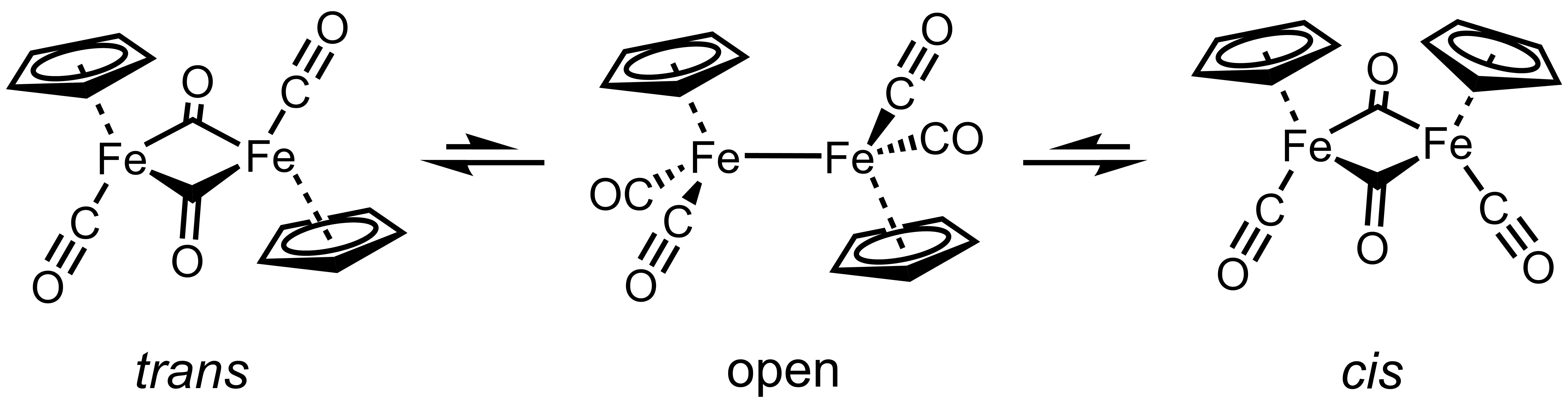 In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridgingŌĆōterminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure
In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridgingŌĆōterminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure

 The alkene ligand in these cations is activated toward attack by
The alkene ligand in these cations is activated toward attack by  Fp(alkene)+ and Fp(alkyne)+ ŽĆ-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral FpŌĆōallyl and FpŌĆōallenyl Žā-complexes (eqn 1).
:(1) + Et3N ŌåÆ FpCH2CH=CHCH3 +
:(2) FpCH2CH=CHCH3 + ŌåÆ
FpŌĆōallyl and FpŌĆōallenyl react with cationic electrophiles E (such as Me3O+, carbocations, oxocarbenium ions) to generate allylic and propargylic functionalization products, respectively (eqn 2). The related complex p*Fe(CO)2(thf)sup>+ F4sup>ŌłÆ has been shown to catalyze propargylic and allylic CŌłÆH functionalization by combining the deprotonation and electrophilic functionalization processes described above.
╬Ę2-Allenyl complexes of Fp+ and substituted cyclopentadienyliron dicarbonyl cations have also been characterized, with X-ray crystallographic analysis showing substantial bending at the central allenic carbon (bond angle < 150┬░).
Fp(alkene)+ and Fp(alkyne)+ ŽĆ-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral FpŌĆōallyl and FpŌĆōallenyl Žā-complexes (eqn 1).
:(1) + Et3N ŌåÆ FpCH2CH=CHCH3 +
:(2) FpCH2CH=CHCH3 + ŌåÆ
FpŌĆōallyl and FpŌĆōallenyl react with cationic electrophiles E (such as Me3O+, carbocations, oxocarbenium ions) to generate allylic and propargylic functionalization products, respectively (eqn 2). The related complex p*Fe(CO)2(thf)sup>+ F4sup>ŌłÆ has been shown to catalyze propargylic and allylic CŌłÆH functionalization by combining the deprotonation and electrophilic functionalization processes described above.
╬Ę2-Allenyl complexes of Fp+ and substituted cyclopentadienyliron dicarbonyl cations have also been characterized, with X-ray crystallographic analysis showing substantial bending at the central allenic carbon (bond angle < 150┬░).

organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
with the formula ''╬Ę''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform
Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various ...
and pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
, but less soluble in carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemi ...
and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below).
Structure
In solution, Cp2Fe2(CO)4 can be considered a dimeric half-sandwich complex. It exists in three isomeric forms: ''cis'', ''trans'', and an unbridged, open form. These isomeric forms are distinguished by the position of the ligands. The ''cis'' and ''trans'' isomers differ in the relative position of C5H5 (Cp) ligands. The ''cis'' and ''trans'' isomers have the formulation ''╬Ę''5-C5H5)Fe(CO)(''╬╝''-CO)sub>2, that is, two CO ligands are terminal whereas the other two CO ligands bridge between the iron atoms. The ''cis'' and ''trans'' isomers interconvert via the open isomer, which has no bridging ligands between iron atoms. Instead, it is formulated as (''╬Ę''5-C5H5)(OC)2FeŌłÆFe(CO)2(''╬Ę''5-C5H5) ŌĆö the metals are held together by an ironŌĆōiron bond. At equilibrium, the ''cis'' and ''trans'' isomers are predominant. : In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridgingŌĆōterminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure
In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridgingŌĆōterminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure fluxional
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in s ...
. The fluxional process for cyclopentadienyliron dicarbonyl dimer is faster than the NMR time scale, so that only an averaged, single Cp signal is observed in the 1H NMR spectrum at 25 ┬░C. Likewise, the 13C NMR spectrum exhibits one sharp CO signal above ŌłÆ10 ┬░C, while the Cp signal sharpens to one peak above 60 ┬░C. NMR studies indicate that the ''cis'' isomer is slightly more abundant than the ''trans'' isomer at room temperature, while the amount of the open form is small. The fluxional process is not fast enough to produce averaging in the IR spectrum
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functio ...
. Thus, three absorptions are seen for each isomer. The bridging CO ligands appear at around 1780 cmŌłÆ1 whereas the terminal CO ligands are observed at around 1980 cmŌłÆ1. The averaged structure of these isomers of Cp2Fe2(CO)4 results in a dipole moment of 3.1 D in benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
.
The solid-state molecular structure of both ''cis'' and ''trans'' isomers have been analyzed by X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
and neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to ob ...
. The FeŌĆōFe separation and the FeŌĆōC bond lengths are the same in the Fe2C2 rhomboids, an exactly planar Fe2C2 four-membered ring in the ''trans'' isomer versus a folded rhomboid in ''cis'' with an angle of 164┬░, and significant distortions in the Cp ring of the ''trans'' isomer reflecting different Cp orbital populations. Although older textbooks show the two iron atoms bonded to each other, theoretical analyses indicate the absence of a direct FeŌĆōFe bond. This view is consistent with computations and X-ray crystallographic data that indicate a lack of significant electron density between the iron atoms. However, Labinger offers a dissenting view, based primarily on chemical reactivity and spectroscopic data, arguing that electron density is not necessarily the best indication of the presence of a chemical bond. Moreover, without an FeŌĆōFe bond, the bridging carbonyls must be formally treated as an ╬╝-X2 ligand and ╬╝-L ligand in order for the iron centers to satisfy the 18-electron rule. This formalism is argued to give misleading implications with respect to the chemical and spectroscopic behavior of the carbonyl groups.Synthesis
Cp2Fe2(CO)4 was first prepared in 1955 at Harvard byGeoffrey Wilkinson
Sir Geoffrey Wilkinson FRS (14 July 1921 ŌĆō 26 September 1996) was a Nobel laureate English chemist who pioneered inorganic chemistry and homogeneous transition metal catalysis.
Education and early life
Wilkinson was born at Springside, Todm ...
using the same method employed today: the reaction of iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to ...
and dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor ...
.
:2 Fe(CO)5 + C10H12 ŌåÆ (''╬Ę''5-C5H5)2Fe2(CO)4 + 6 CO + H2
In this preparation, dicyclopentadiene cracks to give cyclopentadiene, which reacts with Fe(CO)5 with loss of CO. Thereafter, the pathways for the photochemical and thermal routes differ subtly but both entail formation of a hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
intermediate. The method is used in the teaching laboratory.
Reactions
Although of no major commercial value, Fp2 is a workhorse inorganometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
because it is inexpensive and FpX derivatives are rugged (X = halide, organyl).
"FpŌłÆ" (FpNa and FpK)
Reductive cleavage of pFe(CO)2sub>2 (formally an iron(I) complex) produces alkali metal derivatives formally derived from the cyclopentadienyliron dicarbonyl anion, pFe(CO)2sup>ŌłÆ or called FpŌłÆ (formally iron(0)), which are assumed to exist as a tight ion pair. A typical reductant is sodium metal or sodium amalgam;NaK
In data networking, telecommunications, and computer buses, an acknowledgment (ACK) is a signal that is passed between communicating processes, computers, or devices to signify acknowledgment, or receipt of message, as part of a communications ...
alloy, potassium graphite (KC8), and alkali metal trialkylborohydrides have been used. pFe(CO)2a is a widely studied reagent since it is readily alkylated, acylated, or metalated by treatment with an appropriate electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
. It is an excellent SN2 nucleophile, being one to two orders of magnitude more nucleophilic than thiophenolate, PhSŌĆō when reacted with primary and secondary alkyl bromides.
: pFe(CO)2sub>2 + 2 Na ŌåÆ 2 CpFe(CO)2Na
: pFe(CO)2sub>2 + 2 KBH(C2H5)3 ŌåÆ 2 CpFe(CO)2K + H2 + 2 B(C2H5)3
Treatment of NaFp with an alkyl halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a flu ...
(RX, X = Br, I) produces FeR(''╬Ę''5-C5H5)(CO)2
:CpFe(CO)2K + CH3I ŌåÆ CpFe(CO)2CH3 + KI
Fp2 can also be cleaved with alkali metals and by electrochemical reduction.
FpX (X = Cl, Br, I)
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this grou ...
s oxidatively cleave pFe(CO)2sub>2 to give the Fe(II) species FpX (X = Cl, Br, I):
: pFe(CO)2sub>2 + X2 ŌåÆ 2 CpFe(CO)2X
One example is cyclopentadienyliron dicarbonyl iodide.
Fp(''╬Ę''2-alkene)+, Fp(''╬Ę''2-alkyne)+ and other "Fp+"
In the presence of halide anion acceptors such asaluminium bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as al ...
or silver tetrafluoroborate
Silver tetrafluoroborate is an inorganic compound with the chemical formula AgBF4. It is a white solid that dissolves in polar organic solvents as well as water. In its solid state, the Ag+ centers are bound to fluoride.
Preparation
Silver tetraf ...
, FpX compounds (X = halide) react with alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbonŌĆōcarbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
, alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbonŌĆöcarbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
, or neutral labile ligands (such as ether
In organic chemistry, ethers are a class of compounds that contain an ether groupŌĆöan oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and RŌĆ▓ represent the alkyl or aryl groups. Ethers can again ...
s and nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including me ...
s) to afford Fp+ complexes. In another approach, salts of p(isobutene)sup>+ are readily obtained by reaction of NaFp with methallyl chloride
Methallyl chloride is the organic compound with the formula CH2=C(CH3)CH2Cl. It is a colorless liquid and a lacrymator. Its properties are similar to those of allyl chloride. It is a strong alkylating agent
Alkylation is the transfer of an a ...
followed by protonolysis. This complex is a convenient and general precursor to other cationic FpŌĆōalkene and FpŌĆōalkyne complexes. The exchange process is facilitated by the loss of gaseous and bulky isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Produc ...
. Generally, less substituted alkenes bind more strongly and can displace more hindered alkene ligands. Alkene and alkyne complexes can also be prepared by heating a cationic ether or aqua complex, for example , with the alkene or alkyne. complexes can also be prepared by treatment of FpMe with HBF4┬Ę Et2O in CH2Cl2 at ŌłÆ78 ┬░C, followed by addition of L.
:
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbonŌĆōcarbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
ŌĆōFp complexes can also be prepared from Fp anion indirectly. Thus, hydride abstraction from FpŌĆōalkyl compounds using triphenylmethyl hexafluorophosphate affords p(╬▒-alkene)sup>+ complexes.
:FpNa + RCH2CH2I ŌåÆ FpCH2CH2R + NaI
:FpCH2CH2R + Ph3CPF6 ŌåÆ + Ph3CH
Reaction of NaFp with an epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
followed by acid-promoted dehydration also affords alkene complexes. Fp(alkene)+ are stable with respect to bromination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymer ...
, hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
, and acetoxymercuration, but the alkene is easily released with sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I ...
in acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
or by warming with acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile ( hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
.
: The alkene ligand in these cations is activated toward attack by
The alkene ligand in these cations is activated toward attack by nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
s, opening the way to a number of carbonŌĆōcarbon bond-forming reactions. Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions d ...
s usually occur at the more substituted carbon. This regiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
is attributed to the greater positive charge density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter Žü) is the quantity of charge per unit volume, measured in the SI system i ...
at this position. The regiocontrol is often modest. The addition of the nucleophile is completely stereoselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
, occurring ''anti'' to the Fp group. Analogous Fp(alkyne)+ complexes are also reported to undergo nucleophilic addition reactions by various carbon, nitrogen, and oxygen nucleophiles.
: Fp(alkene)+ and Fp(alkyne)+ ŽĆ-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral FpŌĆōallyl and FpŌĆōallenyl Žā-complexes (eqn 1).
:(1) + Et3N ŌåÆ FpCH2CH=CHCH3 +
:(2) FpCH2CH=CHCH3 + ŌåÆ
FpŌĆōallyl and FpŌĆōallenyl react with cationic electrophiles E (such as Me3O+, carbocations, oxocarbenium ions) to generate allylic and propargylic functionalization products, respectively (eqn 2). The related complex p*Fe(CO)2(thf)sup>+ F4sup>ŌłÆ has been shown to catalyze propargylic and allylic CŌłÆH functionalization by combining the deprotonation and electrophilic functionalization processes described above.
╬Ę2-Allenyl complexes of Fp+ and substituted cyclopentadienyliron dicarbonyl cations have also been characterized, with X-ray crystallographic analysis showing substantial bending at the central allenic carbon (bond angle < 150┬░).
Fp(alkene)+ and Fp(alkyne)+ ŽĆ-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral FpŌĆōallyl and FpŌĆōallenyl Žā-complexes (eqn 1).
:(1) + Et3N ŌåÆ FpCH2CH=CHCH3 +
:(2) FpCH2CH=CHCH3 + ŌåÆ
FpŌĆōallyl and FpŌĆōallenyl react with cationic electrophiles E (such as Me3O+, carbocations, oxocarbenium ions) to generate allylic and propargylic functionalization products, respectively (eqn 2). The related complex p*Fe(CO)2(thf)sup>+ F4sup>ŌłÆ has been shown to catalyze propargylic and allylic CŌłÆH functionalization by combining the deprotonation and electrophilic functionalization processes described above.
╬Ę2-Allenyl complexes of Fp+ and substituted cyclopentadienyliron dicarbonyl cations have also been characterized, with X-ray crystallographic analysis showing substantial bending at the central allenic carbon (bond angle < 150┬░).
Fp-based cyclopropanation reagents
Fp-based reagents have been developed forcyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolon ...
s. The key reagent is prepared from FpNa with a thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...
and methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one ...
, and has a good shelf-life, in contrast to typical Simmons-Smith intermediates and diazoalkane
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes ...
s.
:FpNa + ClCH2SCH3 ŌåÆ FpCH2SCH3 + NaCl
:FpCH2SCH3 + CH3I + NaBF4 ŌåÆ FpCH2S(CH3)2]BF4 + NaI
Use of pCH2S(CH3)2F4 does not require specialized conditions.
: + (Ph)2C=CH2 ŌåÆ 1,1-diphenylcyclopropane + ŌĆ”
Iron(III) chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 ┬░C. The col ...
is added to destroy any byproduct.
Precursors to , like FpCH2OMe which is converted to the iron carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" m ...
upon protonation, have also been used as cyclopropanation reagents.
Photochemical reaction
Fp2 exhibitsphotochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet ( wavelength from 100 to 400 nm), visible light (400Ō ...
. For example, upon UV irradiation at 350 nm, it is reduced by benzylnicotinamide, 1-benzyl-1,4-dihydronicotinamide dimer, also known as (BNA)2.
:
References
{{iron compounds Organoiron compounds Carbonyl complexes Cyclopentadienyl complexes Dimers (chemistry) Half sandwich compounds