Charge-transfer salt on:
[Wikipedia]
[Google]
[Amazon]
 In
In

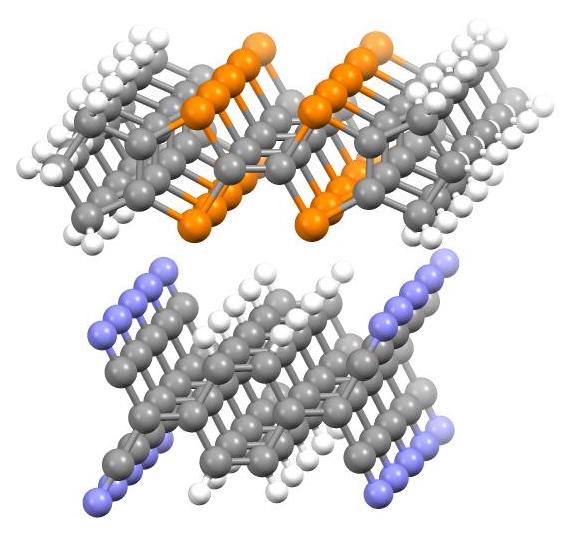 In 1954, charge-transfer salts derived from
In 1954, charge-transfer salts derived from
 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined num ...
of two or more molecules or ions. The assembly consists of two molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s that self-attract through electrostatic
Electrostatics is a branch of physics that studies electric charges at rest ( static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for ...
forces, i.e., one has at least partial negative charge and the partner has partial positive charge, referred to respectively as the electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mista ...
and electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chemi ...
. In some cases, the degree of charge transfer is "complete", such that the CT complex can be classified as a salt. In other cases, the charge-transfer association is weak, and the interaction can be disrupted easily by polar solvents.
Examples
Electron donor-acceptor complexes
A number of organic compounds form charge-transfer complex, which are often described as electron-donor-acceptor complexes (EDA complexes). Typical acceptors are nitrobenzenes or tetracyanoethylene. The strength of their interaction with electron donors correlates with the ionization potentials of the components. For TCNE, the stability constants (L/mol) for its complexes with benzene derivatives correlates with the number of methyl groups:benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
(0.128), 1,3,5-trimethylbenzene (1.11), 1,2,4,5-tetramethylbenzene (3.4), and hexamethylbenzene (16.8).
1,3,5-Trinitrobenzene and related polynitrated aromatic compounds, being electron-deficient, form charge-transfer complexes with many arenes. Such complexes form upon crystallization, but often dissociate in solution to the components. Characteristically, these CT salts crystallize in stacks of alternating donor and acceptor (nitro aromatic) molecules, i.e. A-B-A-B.
Dihalogen/interhalogen CT complexes
Dihalogens X2 (X = Cl, Br, I) and interhalogens XY(X = I; Y = Cl, Br) are Lewis acid species capable of forming a variety of products when reacted with donor species. Among these species (including oxidation or protonated products), CT adducts D·XY have been largely investigated. The CT interaction has been quantified and is the basis of many schemes for parameterizing donor and acceptor properties, such as those devised by Gutmann, Childs, Beckett, and the ECW model. Many organic species featuring chalcogen or pnicogen donor atoms form CT salts. The nature of the resulting adducts can be investigated both in solution and in the solid state. In solution, the intensity of charge-transfer bands in the UV-Vis absorbance spectrum is strongly dependent upon the degree (equilibrium constant) of this association reaction. Methods have been developed to determine the equilibrium constant for these complexes in solution by measuring the intensity of absorption bands as a function of the concentration of donor and acceptor components in solution. The Benesi-Hildebrand method, named for its developers, was first described for the association of iodine dissolved in aromatic hydrocarbons. In the solid state a valuable parameter is the elongation of the X–X or X–Y bond length, resulting from the antibonding nature of the σ* LUMO. The elongation can be evaluated by means of structural determinations (XRD) and FT-Raman spectroscopy. A well-known example is the complex formed byiodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
when combined with starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human die ...
, which exhibits an intense purple charge-transfer band
193px, The intense color of tris(bipyridine)ruthenium(II) arises from a metal-to-ligand charge-transfer band.
Charge-transfer bands are a characteristic feature of the optical spectra of many compounds. These bands are typically more intense tha ...
. This has widespread use as a rough screen for counterfeit currency. Unlike most paper, the paper used in US currency is not sized with starch. Thus, formation of this purple color on application of an iodine solution indicates a counterfeit.
TTF-TCNQ: prototype for electrically conducting complexes

 In 1954, charge-transfer salts derived from
In 1954, charge-transfer salts derived from perylene
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, ...
with iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
or bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
were reported with resistivities as low as 8 ohm·cm. In 1973, it was discovered that a combination tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene
Tetrathiafulvalene is an organosulfur compound with the formula (. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, , by replacement of four CH group ...
(TTF) form a strong charge-transfer complex, referred to as TTF-TCNQ. The solid shows almost metallic electrical conductance and was the first discovered purely organic conductor. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks. Hence, electrons and electron hole
In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is a quasiparticle which is the lack of an electron at a position where one could exist in an atom or atomic lattice. Since in a normal atom or ...
s are separated and concentrated in the stacks and can traverse in a one-dimensional direction along the TCNQ and TTF columns, respectively, when an electric potential is applied to the ends of a crystal in the stack direction.
Superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
is exhibited by tetramethyl-tetraselenafulvalene-hexafluorophosphate (TMTSF2PF6), which is a semi-conductor at ambient conditions, shows superconductivity at low temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
(critical temperature
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
* Critical juncture, a discontinuous change studied in the social sciences.
* Critical Software, a company specializing ...
) and high pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
: 0.9 K and 12 kbar
Bar or BAR may refer to:
Food and drink
* Bar (establishment), selling alcoholic beverages
* Candy bar
* Chocolate bar
Science and technology
* Bar (river morphology), a deposit of sediment
* Bar (tropical cyclone), a layer of cloud
* Bar ( ...
. Critical current densities in these complexes are very small.
Mechanistic implications
Many reactions involving nucleophiles attacking electrophiles can be usefully assessed from the perspective of an incipient charge-transfer complex. Examples includeelectrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
, the addition of Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
s to ketones, and brominolysis of metal-alkyl bonds.
See also
*Organic semiconductor
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or ...
* Organic superconductor
An organic superconductor is a synthetic organic compound that exhibits superconductivity at low temperatures.
As of 2007 the highest achieved critical temperature for an organic superconductor at standard pressure is , observed in the alkali-dop ...
* Exciplex - a special case where one of the molecules is in an excited state
Historical sources
* Y. Okamoto and W. Brenner ''Organic Semiconductors'', Rheinhold (1964) *References
{{DEFAULTSORT:Charge-Transfer Complex Physical organic chemistry Molecular electronics Organic semiconductors