Cadmium sulfate on:
[Wikipedia]
[Google]
[Amazon]
Cadmium sulfate is the name of a series of related
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
s with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
CdSO4·H2O. The most common form is the monohydrate CdSO4·H2O, but two other forms are known CdSO4·H2O and the anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
salt (CdSO4). All salts are colourless and highly soluble in water.
Structure, preparation, and occurrence

X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
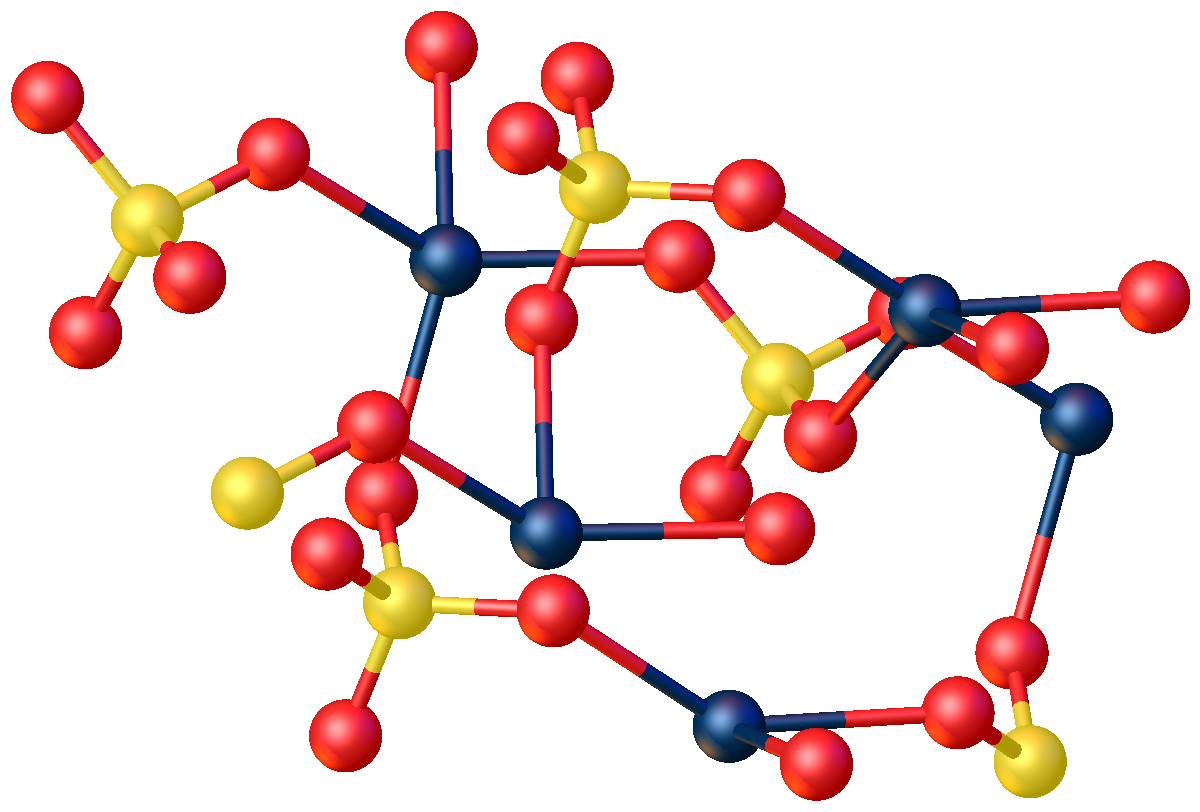
shows that CdSO4·H2O is a typical coordination polymer. Each Cd2+ center has octahedral coordination geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc ...
, being surrounded by four oxygen centers provided by four sulfate ligands and two oxygen centers from the bridging water ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s.
Cadmium sulfate hydrate can be prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid:
: CdO + H2SO4 → CdSO4 + H2O
: Cd + H2SO4 → CdSO4 + H2
The anhydrous material can be prepared using sodium persulfate
Sodium persulfate is the inorganic compound with the formula Sodium, Na2Sulfur, S2Oxygen, O8. It is the sodium salt of peroxydisulfuric acid, H2S2O8, an oxidizing agent. It is a white solid that dissolves in water. It is almost non-hygroscopic an ...
:
: Cd + Na2S2O8 → CdSO4 + Na2SO4
Cadmium sulfates occur as the following rare minerals drobecite (CdSO4·4H2O), voudourisite (monohydrate), and lazaridisite (the 8/3-hydrate).
Applications
Cadmium sulfate is used widely for the electroplating of cadmium in electronic circuits. It is also a precursor to cadmium-based pigment such ascadmium sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid.Egon Wiberg, Arnold Frederick Holleman (2001''Inorganic Chemistry'' Elsevier It occurs in nature with two different crystal structures as the rare m ...
. It is also used for electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
in a Weston standard cell
The Weston standard cell is a wet-chemical cell that produces a highly stable voltage suitable as a laboratory standard for calibration of voltmeters. Invented by Edward Weston in 1893, it was adopted as the International Standard for EMF fro ...
as well as a pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compo ...
in fluorescent screens.
References