Brownian Motion (Ultimate) on:
[Wikipedia]
[Google]
[Amazon]
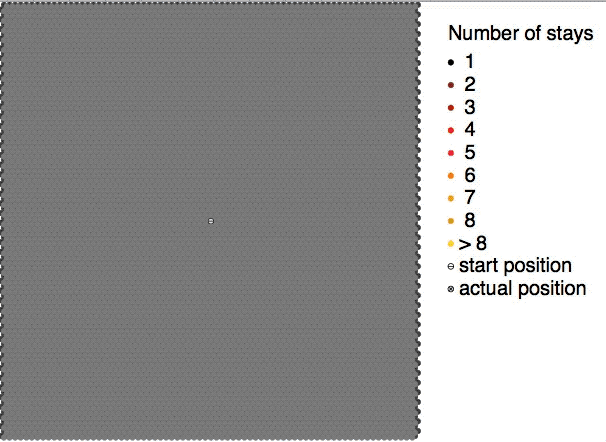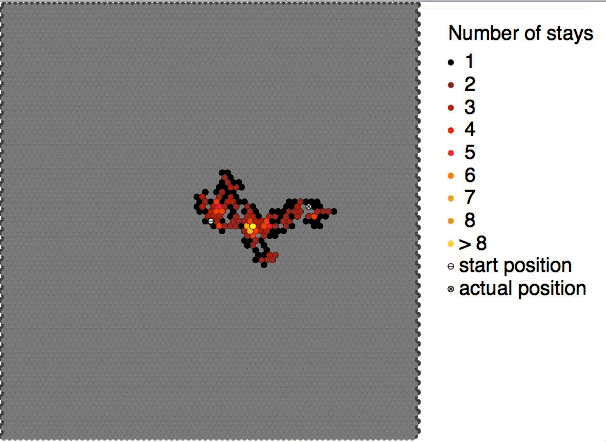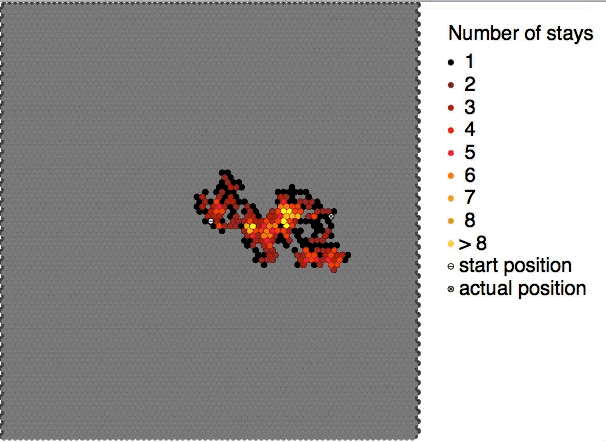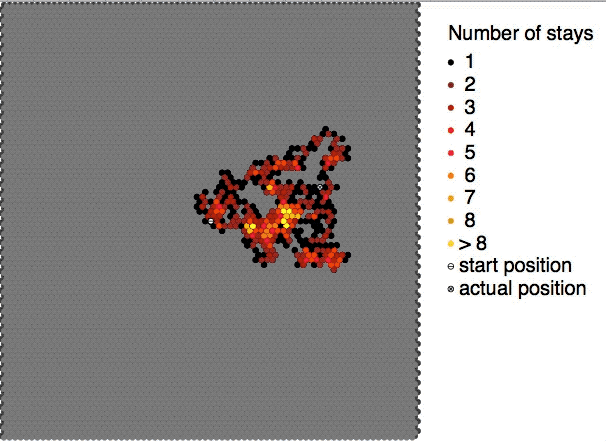
 Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of
 The Roman philosopher-poet
The Roman philosopher-poet
 The first part of Einstein's argument was to determine how far a Brownian particle travels in a given time interval. Classical mechanics is unable to determine this distance because of the enormous number of bombardments a Brownian particle will undergo, roughly of the order of 1014 collisions per second.
He regarded the increment of particle positions in time in a one-dimensional (''x'') space (with the coordinates chosen so that the origin lies at the initial position of the particle) as a random variable () with some
The first part of Einstein's argument was to determine how far a Brownian particle travels in a given time interval. Classical mechanics is unable to determine this distance because of the enormous number of bombardments a Brownian particle will undergo, roughly of the order of 1014 collisions per second.
He regarded the increment of particle positions in time in a one-dimensional (''x'') space (with the coordinates chosen so that the origin lies at the initial position of the particle) as a random variable () with some 
 The Wiener process ''Wt'' is characterized by four facts:
# ''W''0 = 0
# ''Wt'' is
The Wiener process ''Wt'' is characterized by four facts:
# ''W''0 = 0
# ''Wt'' is
 The Infinitesimal generator (stochastic processes), infinitesimal generator (and hence characteristic operator) of a Brownian motion on R''n'' is easily calculated to be ½Δ, where Δ denotes the Laplace operator. In image processing and computer vision, the Laplacian operator has been used for various tasks such as blob and edge detection. This observation is useful in defining Brownian motion on an ''m''-dimensional Riemannian manifold (''M'', ''g''): a Brownian motion on ''M'' is defined to be a diffusion on ''M'' whose characteristic operator in local coordinates ''x''''i'', 1 ≤ ''i'' ≤ ''m'', is given by ½ΔLB, where ΔLB is the Laplace–Beltrami operator given in local coordinates by
:
where [''g''''ij''] = [''g''''ij'']−1 in the sense of Invertible matrix, the inverse of a square matrix.
The Infinitesimal generator (stochastic processes), infinitesimal generator (and hence characteristic operator) of a Brownian motion on R''n'' is easily calculated to be ½Δ, where Δ denotes the Laplace operator. In image processing and computer vision, the Laplacian operator has been used for various tasks such as blob and edge detection. This observation is useful in defining Brownian motion on an ''m''-dimensional Riemannian manifold (''M'', ''g''): a Brownian motion on ''M'' is defined to be a diffusion on ''M'' whose characteristic operator in local coordinates ''x''''i'', 1 ≤ ''i'' ≤ ''m'', is given by ½ΔLB, where ΔLB is the Laplace–Beltrami operator given in local coordinates by
:
where [''g''''ij''] = [''g''''ij'']−1 in the sense of Invertible matrix, the inverse of a square matrix.
on-line version
', from Project Gutenberg. See the heading 'Atomic Motions'; this translation differs slightly from the one quoted). * Edward Nelson, Nelson, Edward, (1967). ''Dynamical Theories of Brownian Motion''
(PDF version of this out-of-print book, from the author's webpage.)
This is primarily a mathematical work, but the first four chapters discuss the history of the topic, in the era from Brown to Einstein. * * ** See also Perrin's book "Les Atomes" (1914). * * * Thorvald N. Thiele, Theile, T. N. ** Danish version: "Om Anvendelse af mindste Kvadraters Methode i nogle Tilfælde, hvor en Komplikation af visse Slags uensartede tilfældige Fejlkilder giver Fejlene en 'systematisk' Karakter". ** French version: "Sur la compensation de quelques erreurs quasi-systématiques par la méthodes de moindre carrés" published simultaneously in ''Vidensk. Selsk. Skr. 5. Rk., naturvid. og mat. Afd.'', 12:381–408, 1880.
Einstein on Brownian Motion
* [http://www.gizmag.com/einsteins-prediction-finally-witnessed/16212/ "Einstein's prediction finally witnessed one century later"] : a test to observe the velocity of Brownian motion
Large-Scale Brownian Motion Demonstration
{{Authority control Statistical mechanics Wiener process Fractals Colloidal chemistry Robert Brown (botanist, born 1773) Albert Einstein Articles containing video clips Lévy processes

 Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particle
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from ...
s suspended in a medium (a liquid or a gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
).
This pattern of motion typically consists of random
In common usage, randomness is the apparent or actual lack of pattern or predictability in events. A random sequence of events, symbols or steps often has no order and does not follow an intelligible pattern or combination. Individual ra ...
fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be i ...
, defined by a given temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mecha ...
). More specifically, the fluid's overall linear
Linearity is the property of a mathematical relationship ('' function'') that can be graphically represented as a straight line. Linearity is closely related to '' proportionality''. Examples in physics include rectilinear motion, the linear ...
and angular momenta remain null over time. The kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's internal energy (the equipartition theorem
In classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. T ...
).
This motion is named after the botanist Robert Brown, who first described the phenomenon in 1827, while looking through a microscope at pollen of the plant '' Clarkia pulchella'' immersed in water. In 1905, almost eighty years later, theoretical physicist Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
published a paper where he modeled the motion of the pollen particles as being moved by individual water molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s, making one of his first major scientific contributions. The direction of the force of atomic bombardment is constantly changing, and at different times the particle is hit more on one side than another, leading to the seemingly random nature of the motion. This explanation of Brownian motion served as convincing evidence that atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s and molecules exist and was further verified experimentally by Jean Perrin in 1908. Perrin was awarded the Nobel Prize in Physics
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then " ...
in 1926 "for his work on the discontinuous structure of matter".
The many-body interactions that yield the Brownian pattern cannot be solved by a model accounting for every involved molecule. In consequence, only probabilistic models applied to molecular populations can be employed to describe it. Two such models of the statistical mechanics, due to Einstein and Smoluchowski, are presented below. Another, pure probabilistic class of models is the class of the stochastic process models. There exist sequences of both simpler and more complicated stochastic processes which converge (in the limit) to Brownian motion (see random walk
In mathematics, a random walk is a random process that describes a path that consists of a succession of random steps on some mathematical space.
An elementary example of a random walk is the random walk on the integer number line \mathbb Z ...
and Donsker's theorem
In probability theory, Donsker's theorem (also known as Donsker's invariance principle, or the functional central limit theorem), named after Monroe D. Donsker, is a functional extension of the central limit theorem.
Let X_1, X_2, X_3, \ldots be ...
).
History
Lucretius
Titus Lucretius Carus ( , ; – ) was a Roman poet and philosopher. His only known work is the philosophical poem ''De rerum natura'', a didactic work about the tenets and philosophy of Epicureanism, and which usually is translated into En ...
' scientific poem "On the Nature of Things
''De rerum natura'' (; ''On the Nature of Things'') is a first-century BC didactic poem by the Roman poet and philosopher Lucretius ( – c. 55 BC) with the goal of explaining Epicurean philosophy to a Roman audience. The poem, written in some 7 ...
" (c. 60 BC) has a remarkable description of the motion of dust
Dust is made of fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian process), volcanic eruptions, and pollution. Dust in ho ...
particles in verses 113–140 from Book II. He uses this as a proof of the existence of atoms:
Although the mingling, tumbling motion of dust particles is caused largely by air currents, the glittering, jiggling motion of small dust particles is caused chiefly by true Brownian dynamics; Lucretius "perfectly describes and explains the Brownian movement by a wrong example".
While Jan Ingenhousz
Jan (or John) Ingenhousz or Ingen-Housz FRS (8 December 1730 – 7 September 1799) was a Dutch-born British physiologist, biologist and chemist.
He is best known for discovering photosynthesis by showing that light is essential to the process ...
described the irregular motion of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when ...
dust
Dust is made of fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian process), volcanic eruptions, and pollution. Dust in ho ...
particles on the surface of alcohol in 1785, the discovery of this phenomenon is often credited to the botanist Robert Brown in 1827. Brown was studying pollen grains of the plant '' Clarkia pulchella'' suspended in water under a microscope when he observed minute particles, ejected by the pollen grains, executing a jittery motion. By repeating the experiment with particles of inorganic matter he was able to rule out that the motion was life-related, although its origin was yet to be explained.
The first person to describe the mathematics behind Brownian motion was Thorvald N. Thiele
Thorvald Nicolai Thiele (24 December 1838 – 26 September 1910) was a Danish astronomer and director of the Copenhagen Observatory. He was also an actuary and mathematician, most notable for his work in statistics, interpolation and the three- ...
in a paper on the method of least squares published in 1880. This was followed independently by Louis Bachelier
Louis Jean-Baptiste Alphonse Bachelier (; 11 March 1870 – 28 April 1946) was a French mathematician at the turn of the 20th century. He is credited with being the first person to model the stochastic process now called Brownian motion, as part ...
in 1900 in his PhD thesis "The theory of speculation", in which he presented a stochastic analysis of the stock and option markets. The Brownian motion model of the stock market is often cited, but Benoit Mandelbrot rejected its applicability to stock price movements in part because these are discontinuous.
Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
(in one of his 1905 papers) and Marian Smoluchowski (1906) brought the solution of the problem to the attention of physicists, and presented it as a way to indirectly confirm the existence of atoms and molecules. Their equations describing Brownian motion were subsequently verified by the experimental work of Jean Baptiste Perrin
Jean Baptiste Perrin (30 September 1870 – 17 April 1942) was a French physicist who, in his studies of the Brownian motion of minute particles suspended in liquids (sedimentation equilibrium), verified Albert Einstein’s explanation of this p ...
in 1908.
Statistical mechanics theories
Einstein's theory
There are two parts to Einstein's theory: the first part consists in the formulation of a diffusion equation for Brownian particles, in which the diffusion coefficient is related to themean squared displacement
In statistical mechanics, the mean squared displacement (MSD, also mean square displacement, average squared displacement, or mean square fluctuation) is a measure of the deviation of the position of a particle with respect to a reference positi ...
of a Brownian particle, while the second part consists in relating the diffusion coefficient to measurable physical quantities. In this way Einstein was able to determine the size of atoms, and how many atoms there are in a mole, or the molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
in grams, of a gas. In accordance to Avogadro's law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific c ...
, this volume is the same for all ideal gases, which is 22.414 liters at standard temperature and pressure. The number of atoms contained in this volume is referred to as the Avogadro number
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining co ...
, and the determination of this number is tantamount to the knowledge of the mass of an atom, since the latter is obtained by dividing the molar mass
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance which is the number of moles in that sample, measured in moles. The molar mass is a bulk, not molecular, ...
of the gas by the Avogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining c ...
.
probability density function
In probability theory, a probability density function (PDF), or density of a continuous random variable, is a function whose value at any given sample (or point) in the sample space (the set of possible values taken by the random variable) ca ...
(i.e., is the probability density for a jump of magnitude , i.e., the probability density of the particle incrementing its position from to in the time interval ). Further, assuming conservation of particle number, he expanded the number density (number of particles per unit volume around ) at time in a Taylor series
In mathematics, the Taylor series or Taylor expansion of a function is an infinite sum of terms that are expressed in terms of the function's derivatives at a single point. For most common functions, the function and the sum of its Taylor ser ...
,
where the second equality is by definition of . The integral
In mathematics, an integral assigns numbers to functions in a way that describes displacement, area, volume, and other concepts that arise by combining infinitesimal data. The process of finding integrals is called integration. Along wit ...
in the first term is equal to one by the definition of probability, and the second and other even terms (i.e. first and other odd moments) vanish because of space symmetry. What is left gives rise to the following relation:
Where the coefficient after the Laplacian, the second moment of probability of displacement , is interpreted as mass diffusivity
Diffusivity, mass diffusivity or diffusion coefficient is a proportionality constant between the molar flux due to molecular diffusion and the gradient in the concentration of the species (or the driving force for diffusion). Diffusivity is enco ...
''D'':
Then the density of Brownian particles ''ρ'' at point ''x'' at time ''t'' satisfies the diffusion equation:
Assuming that ''N'' particles start from the origin at the initial time ''t'' = 0, the diffusion equation has the solution
This expression (which is a normal distribution
In statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is
:
f(x) = \frac e^
The parameter \mu ...
with the mean and variance usually called Brownian motion ) allowed Einstein to calculate the moments directly. The first moment is seen to vanish, meaning that the Brownian particle is equally likely to move to the left as it is to move to the right. The second moment is, however, non-vanishing, being given by
This equation expresses the mean squared displacement in terms of the time elapsed and the diffusivity. From this expression Einstein argued that the displacement of a Brownian particle is not proportional to the elapsed time, but rather to its square root. His argument is based on a conceptual switch from the "ensemble" of Brownian particles to the "single" Brownian particle: we can speak of the relative number of particles at a single instant just as well as of the time it takes a Brownian particle to reach a given point.
The second part of Einstein's theory relates the diffusion constant to physically measurable quantities, such as the mean squared displacement of a particle in a given time interval. This result enables the experimental determination of the Avogadro number and therefore the size of molecules. Einstein analyzed a dynamic equilibrium being established between opposing forces. The beauty of his argument is that the final result does not depend upon which forces are involved in setting up the dynamic equilibrium.
In his original treatment, Einstein considered an osmotic pressure experiment, but the same conclusion can be reached in other ways.
Consider, for instance, particles suspended in a viscous fluid in a gravitational field. Gravity tends to make the particles settle, whereas diffusion acts to homogenize them, driving them into regions of smaller concentration. Under the action of gravity, a particle acquires a downward speed of ''v'' = ''μmg'', where ''m'' is the mass of the particle, ''g'' is the acceleration due to gravity, and ''μ'' is the particle's mobility
Mobility may refer to:
Social sciences and humanities
* Economic mobility, ability of individuals or families to improve their economic status
* Geographic mobility, the measure of how populations and goods move over time
* Mobilities, a conte ...
in the fluid. George Stokes had shown that the mobility for a spherical particle with radius ''r'' is , where ''η'' is the dynamic viscosity of the fluid. In a state of dynamic equilibrium, and under the hypothesis of isothermal fluid, the particles are distributed according to the barometric distribution
where ''ρ'' − ''ρ''o is the difference in density of particles separated by a height difference, of , ''k''B is the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
(the ratio of the universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
, ''R'', to the Avogadro constant, ''N''), and ''T'' is the absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic w ...
.

Dynamic equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances transition between the reactants and products at equal rates, meaning there is no net change. Reactants and products are formed at such a rate that the co ...
is established because the more that particles are pulled down by gravity
In physics, gravity () is a fundamental interaction which causes mutual attraction between all things with mass or energy. Gravity is, by far, the weakest of the four fundamental interactions, approximately 1038 times weaker than the stro ...
, the greater the tendency for the particles to migrate to regions of lower concentration. The flux is given by Fick's law
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
,
where ''J'' = ''ρv''. Introducing the formula for ''ρ'', we find that
In a state of dynamical equilibrium, this speed must also be equal to ''v'' = ''μmg''. Both expressions for ''v'' are proportional to ''mg'', reflecting that the derivation is independent of the type of forces considered. Similarly, one can derive an equivalent formula for identical charged particle
In physics, a charged particle is a particle with an electric charge. It may be an ion, such as a molecule or atom with a surplus or deficit of electrons relative to protons. It can also be an electron or a proton, or another elementary pa ...
s of charge ''q'' in a uniform electric field of magnitude ''E'', where ''mg'' is replaced with the electrostatic force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is convention ...
''qE''. Equating these two expressions yields the Einstein relation for the diffusivity, independent of ''mg'' or ''qE'' or other such forces:
Here the first equality follows from the first part of Einstein's theory, the third equality follows from the definition of the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant, ...
as ''k''B = ''R'' / ''N'', and the fourth equality follows from Stokes's formula for the mobility. By measuring the mean squared displacement over a time interval along with the universal gas constant ''R'', the temperature ''T'', the viscosity ''η'', and the particle radius ''r'', the Avogadro constant ''N'' can be determined.
The type of dynamical equilibrium proposed by Einstein was not new. It had been pointed out previously by J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
In 1897, Thomson showed that ...
in his series of lectures at Yale University in May 1903 that the dynamic equilibrium between the velocity generated by a concentration gradient
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) o ...
given by Fick's law and the velocity due to the variation of the partial pressure caused when ions are set in motion "gives us a method of determining Avogadro's Constant which is independent of any hypothesis as to the shape or size of molecules, or of the way in which they act upon each other".
An identical expression to Einstein's formula for the diffusion coefficient was also found by Walther Nernst in 1888 in which he expressed the diffusion coefficient as the ratio of the osmotic pressure to the ratio of the frictional force
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction:
*Dry friction is a force that opposes the relative lateral motion of ...
and the velocity to which it gives rise. The former was equated to the law of van 't Hoff while the latter was given by Stokes's law. He writes for the diffusion coefficient ''k, where is the osmotic pressure and ''k'' is the ratio of the frictional force to the molecular viscosity which he assumes is given by Stokes's formula for the viscosity. Introducing the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stat ...
per unit volume for the osmotic pressure, the formula becomes identical to that of Einstein's. The use of Stokes's law in Nernst's case, as well as in Einstein and Smoluchowski, is not strictly applicable since it does not apply to the case where the radius of the sphere is small in comparison with the mean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a ...
.
At first, the predictions of Einstein's formula were seemingly refuted by a series of experiments by Svedberg in 1906 and 1907, which gave displacements of the particles as 4 to 6 times the predicted value, and by Henri in 1908 who found displacements 3 times greater than Einstein's formula predicted. But Einstein's predictions were finally confirmed in a series of experiments carried out by Chaudesaigues in 1908 and Perrin in 1909. The confirmation of Einstein's theory constituted empirical progress for the kinetic theory of heat. In essence, Einstein showed that the motion can be predicted directly from the kinetic model of thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be i ...
. The importance of the theory lay in the fact that it confirmed the kinetic theory's account of the second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unles ...
as being an essentially statistical law.
Smoluchowski model
Smoluchowski's theory of Brownian motion starts from the same premise as that of Einstein and derives the same probability distribution ''ρ''(''x'', ''t'') for the displacement of a Brownian particle along the ''x'' in time ''t''. He therefore gets the same expression for the mean squared displacement: . However, when he relates it to a particle of mass ''m'' moving at a velocity which is the result of a frictional force governed by Stokes's law, he finds : where ''μ'' is the viscosity coefficient, and is the radius of the particle. Associating the kinetic energy with the thermal energy ''RT''/''N'', the expression for the mean squared displacement is 64/27 times that found by Einstein. The fraction 27/64 was commented on by Arnold Sommerfeld in his necrology on Smoluchowski: "The numerical coefficient of Einstein, which differs from Smoluchowski by 27/64 can only be put in doubt." Smoluchowski attempts to answer the question of why a Brownian particle should be displaced by bombardments of smaller particles when the probabilities for striking it in the forward and rear directions are equal. If the probability of ''m'' gains and ''n'' − ''m'' losses follows a binomial distribution, : with equal ''a priori'' probabilities of 1/2, the mean total gain is : If ''n'' is large enough so that Stirling's approximation can be used in the form : then the expected total gain will be : showing that it increases as the square root of the total population. Suppose that a Brownian particle of mass ''M'' is surrounded by lighter particles of mass ''m'' which are traveling at a speed ''u''. Then, reasons Smoluchowski, in any collision between a surrounding and Brownian particles, the velocity transmitted to the latter will be ''mu''/''M''. This ratio is of the order of 10−7 cm/s. But we also have to take into consideration that in a gas there will be more than 1016 collisions in a second, and even greater in a liquid where we expect that there will be 1020 collision in one second. Some of these collisions will tend to accelerate the Brownian particle; others will tend to decelerate it. If there is a mean excess of one kind of collision or the other to be of the order of 108 to 1010 collisions in one second, then velocity of the Brownian particle may be anywhere between 10 and 1000 cm/s. Thus, even though there are equal probabilities for forward and backward collisions there will be a net tendency to keep the Brownian particle in motion, just as the ballot theorem predicts. These orders of magnitude are not exact because they don't take into consideration the velocity of the Brownian particle, ''U'', which depends on the collisions that tend to accelerate and decelerate it. The larger ''U'' is, the greater will be the collisions that will retard it so that the velocity of a Brownian particle can never increase without limit. Could such a process occur, it would be tantamount to a perpetual motion of the second type. And since equipartition of energy applies, the kinetic energy of the Brownian particle, , will be equal, on the average, to the kinetic energy of the surrounding fluid particle, . In 1906 Smoluchowski published a one-dimensional model to describe a particle undergoing Brownian motion. The model assumes collisions with ''M'' ≫ ''m'' where ''M'' is the test particle's mass and ''m'' the mass of one of the individual particles composing the fluid. It is assumed that the particle collisions are confined to one dimension and that it is equally probable for the test particle to be hit from the left as from the right. It is also assumed that every collision always imparts the same magnitude of Δ''V''. If ''N''R is the number of collisions from the right and ''N''L the number of collisions from the left then after ''N'' collisions the particle's velocity will have changed by Δ''V''(2''N''R − ''N''). Themultiplicity
Multiplicity may refer to: In science and the humanities
* Multiplicity (mathematics), the number of times an element is repeated in a multiset
* Multiplicity (philosophy), a philosophical concept
* Multiplicity (psychology), having or using mult ...
is then simply given by:
:
and the total number of possible states is given by 2''N''. Therefore, the probability of the particle being hit from the right ''NR'' times is:
:
As a result of its simplicity, Smoluchowski's 1D model can only qualitatively describe Brownian motion. For a realistic particle undergoing Brownian motion in a fluid, many of the assumptions don't apply. For example, the assumption that on average occurs an equal number of collisions from the right as from the left falls apart once the particle is in motion. Also, there would be a distribution of different possible Δ''V''s instead of always just one in a realistic situation.
Other physics models using partial differential equations
The diffusion equation yields an approximation of the time evolution of theprobability density function
In probability theory, a probability density function (PDF), or density of a continuous random variable, is a function whose value at any given sample (or point) in the sample space (the set of possible values taken by the random variable) ca ...
associated to the position of the particle going under a Brownian movement under the physical definition. The approximation is valid on short timescales.
The time evolution of the position of the Brownian particle itself is best described using Langevin equation, an equation which involves a random force field representing the effect of the thermal fluctuations
In statistical mechanics, thermal fluctuations are random deviations of a system from its average state, that occur in a system at equilibrium.In statistical mechanics they are often simply referred to as fluctuations. All thermal fluctuations b ...
of the solvent on the particle.
The displacement of a particle undergoing Brownian motion is obtained by solving the diffusion equation under appropriate boundary conditions and finding the rms of the solution. This shows that the displacement varies as the square root of the time (not linearly), which explains why previous experimental results concerning the velocity of Brownian particles gave nonsensical results. A linear time dependence was incorrectly assumed.
At very short time scales, however, the motion of a particle is dominated by its inertia and its displacement will be linearly dependent on time: Δ''x'' = ''v''Δ''t''. So the instantaneous velocity of the Brownian motion can be measured as ''v'' = Δ''x''/Δ''t'', when Δ''t'' << ''τ'', where ''τ'' is the momentum relaxation time. In 2010, the instantaneous velocity of a Brownian particle (a glass microsphere trapped in air with optical tweezers) was measured successfully. The velocity data verified the Maxwell–Boltzmann velocity distribution, and the equipartition theorem for a Brownian particle.
Astrophysics: star motion within galaxies
In stellar dynamics, a massive body (star, black hole, etc.) can experience Brownian motion as it responds to gravitational forces from surrounding stars. The rms velocity ''V'' of the massive object, of mass ''M'', is related to the rms velocity of the background stars by : where is the mass of the background stars. The gravitational force from the massive object causes nearby stars to move faster than they otherwise would, increasing both and ''V''. The Brownian velocity ofSgr A*
Sagittarius A* ( ), abbreviated Sgr A* ( ), is the supermassive black hole at the Galactic Center of the Milky Way. It is located near the border of the constellations Sagittarius and Scorpius, about 5.6° south of the ecliptic, vi ...
, the supermassive black hole
A supermassive black hole (SMBH or sometimes SBH) is the largest type of black hole, with its mass being on the order of hundreds of thousands, or millions to billions of times the mass of the Sun (). Black holes are a class of astronomical ob ...
at the center of the Milky Way galaxy
The Milky Way is the galaxy that includes our Solar System, with the name describing the galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars that cannot be individually distinguished by the naked eye. ...
, is predicted from this formula to be less than 1 km s−1.
Mathematics
In mathematics, Brownian motion is described by the Wiener process, a continuous-time stochastic process named in honor of Norbert Wiener. It is one of the best knownLévy process
In probability theory, a Lévy process, named after the French mathematician Paul Lévy, is a stochastic process with independent, stationary increments: it represents the motion of a point whose successive displacements are random, in which disp ...
es (càdlàg In mathematics, a càdlàg (French: "''continue à droite, limite à gauche''"), RCLL ("right continuous with left limits"), or corlol ("continuous on (the) right, limit on (the) left") function is a function defined on the real numbers (or a subset ...
stochastic processes with stationary independent increments) and occurs frequently in pure and applied mathematics, economics
Economics () is the social science that studies the production, distribution, and consumption of goods and services.
Economics focuses on the behaviour and interactions of economic agents and how economies work. Microeconomics analyzes ...
and physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
.
 The Wiener process ''Wt'' is characterized by four facts:
# ''W''0 = 0
# ''Wt'' is
The Wiener process ''Wt'' is characterized by four facts:
# ''W''0 = 0
# ''Wt'' is almost surely
In probability theory, an event is said to happen almost surely (sometimes abbreviated as a.s.) if it happens with probability 1 (or Lebesgue measure 1). In other words, the set of possible exceptions may be non-empty, but it has probability 0 ...
continuous
# ''Wt'' has independent increments
# (for ).
denotes the normal distribution
In statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is
:
f(x) = \frac e^
The parameter \mu ...
with expected value ''μ'' and variance
In probability theory and statistics, variance is the expectation of the squared deviation of a random variable from its population mean or sample mean. Variance is a measure of dispersion, meaning it is a measure of how far a set of numbe ...
''σ''2. The condition that it has independent increments means that if then and are independent random variables. In addition, for some filtration , is measurable
In mathematics, the concept of a measure is a generalization and formalization of geometrical measures (length, area, volume) and other common notions, such as mass and probability of events. These seemingly distinct concepts have many simila ...
for all .
An alternative characterisation of the Wiener process is the so-called ''Lévy characterisation'' that says that the Wiener process is an almost surely continuous martingale with ''W''0 = 0 and quadratic variation In mathematics, quadratic variation is used in the analysis of stochastic processes such as Brownian motion and other martingales. Quadratic variation is just one kind of variation of a process.
Definition
Suppose that X_t is a real-valued sto ...
.
A third characterisation is that the Wiener process has a spectral representation as a sine series whose coefficients are independent random variables. This representation can be obtained using the Karhunen–Loève theorem.
The Wiener process can be constructed as the scaling limit of a random walk
In mathematics, a random walk is a random process that describes a path that consists of a succession of random steps on some mathematical space.
An elementary example of a random walk is the random walk on the integer number line \mathbb Z ...
, or other discrete-time stochastic processes with stationary independent increments. This is known as Donsker's theorem
In probability theory, Donsker's theorem (also known as Donsker's invariance principle, or the functional central limit theorem), named after Monroe D. Donsker, is a functional extension of the central limit theorem.
Let X_1, X_2, X_3, \ldots be ...
. Like the random walk, the Wiener process is recurrent in one or two dimensions (meaning that it returns almost surely to any fixed neighborhood of the origin infinitely often) whereas it is not recurrent in dimensions three and higher. Unlike the random walk, it is scale invariant
In physics, mathematics and statistics, scale invariance is a feature of objects or laws that do not change if scales of length, energy, or other variables, are multiplied by a common factor, and thus represent a universality.
The technical ter ...
.
The time evolution of the position of the Brownian particle itself can be described approximately by a Langevin equation, an equation which involves a random force field representing the effect of the thermal fluctuations
In statistical mechanics, thermal fluctuations are random deviations of a system from its average state, that occur in a system at equilibrium.In statistical mechanics they are often simply referred to as fluctuations. All thermal fluctuations b ...
of the solvent on the Brownian particle. On long timescales, the mathematical Brownian motion is well described by a Langevin equation. On small timescales, inertia
Inertia is the idea that an object will continue its current motion until some force causes its speed or direction to change. The term is properly understood as shorthand for "the principle of inertia" as described by Newton in his first law ...
l effects are prevalent in the Langevin equation. However the mathematical ''Brownian motion'' is exempt of such inertial effects. Inertial effects have to be considered in the Langevin equation, otherwise the equation becomes singular. so that simply removing the inertia
Inertia is the idea that an object will continue its current motion until some force causes its speed or direction to change. The term is properly understood as shorthand for "the principle of inertia" as described by Newton in his first law ...
term from this equation would not yield an exact description, but rather a singular behavior in which the particle doesn't move at all.
Statistics
The Brownian motion can be modeled by a random walk. In the general case, Brownian motion is aMarkov process
A Markov chain or Markov process is a stochastic model describing a sequence of possible events in which the probability of each event depends only on the state attained in the previous event. Informally, this may be thought of as, "What happe ...
and described by stochastic integral equations.
Lévy characterisation
The French mathematician Paul Lévy proved the following theorem, which gives a necessary and sufficient condition for a continuous R''n''-valued stochastic process ''X'' to actually be ''n''-dimensional Brownian motion. Hence, Lévy's condition can actually be used as an alternative definition of Brownian motion. Let ''X'' = (''X''1, ..., ''X''''n'') be a continuous stochastic process on aprobability space
In probability theory, a probability space or a probability triple (\Omega, \mathcal, P) is a mathematical construct that provides a formal model of a random process or "experiment". For example, one can define a probability space which models t ...
(Ω, Σ, P) taking values in R''n''. Then the following are equivalent:
# ''X'' is a Brownian motion with respect to P, i.e., the law of ''X'' with respect to P is the same as the law of an ''n''-dimensional Brownian motion, i.e., the push-forward measure In measure theory, a pushforward measure (also known as push forward, push-forward or image measure) is obtained by transferring ("pushing forward") a measure from one measurable space to another using a measurable function.
Definition
Given meas ...
''X''∗(P) is classical Wiener measure on ''C''0([0, +∞); R''n'').
# both
## ''X'' is a martingale with respect to P (and its own natural filtration); and
## for all 1 ≤ ''i'', ''j'' ≤ ''n'', ''X''''i''(''t'')''X''''j''(''t'') −''δ''''ij''''t'' is a martingale with respect to P (and its own natural filtration), where ''δ''''ij'' denotes the Kronecker delta.
Spectral content
The spectral content of a stochastic process can be found from the power spectral density, formally defined as where stands for the expected value. The power spectral density of Brownian motion is found to be where is the diffusion coefficient of . For naturally occurring signals, the spectral content can be found from the power spectral density of a single realization, with finite available time, i.e., which for an individual realization of a Brownian motion trajectory, it is found to have expected value andvariance
In probability theory and statistics, variance is the expectation of the squared deviation of a random variable from its population mean or sample mean. Variance is a measure of dispersion, meaning it is a measure of how far a set of numbe ...
For sufficiently long realization times, the expected value of the power spectrum of a single trajectory converges to the formally defined power spectral density , but its coefficient of variation tends to . This implies the distribution of is broad even in the infinite time limit.
Riemannian manifold
 The Infinitesimal generator (stochastic processes), infinitesimal generator (and hence characteristic operator) of a Brownian motion on R''n'' is easily calculated to be ½Δ, where Δ denotes the Laplace operator. In image processing and computer vision, the Laplacian operator has been used for various tasks such as blob and edge detection. This observation is useful in defining Brownian motion on an ''m''-dimensional Riemannian manifold (''M'', ''g''): a Brownian motion on ''M'' is defined to be a diffusion on ''M'' whose characteristic operator in local coordinates ''x''''i'', 1 ≤ ''i'' ≤ ''m'', is given by ½ΔLB, where ΔLB is the Laplace–Beltrami operator given in local coordinates by
:
where [''g''''ij''] = [''g''''ij'']−1 in the sense of Invertible matrix, the inverse of a square matrix.
The Infinitesimal generator (stochastic processes), infinitesimal generator (and hence characteristic operator) of a Brownian motion on R''n'' is easily calculated to be ½Δ, where Δ denotes the Laplace operator. In image processing and computer vision, the Laplacian operator has been used for various tasks such as blob and edge detection. This observation is useful in defining Brownian motion on an ''m''-dimensional Riemannian manifold (''M'', ''g''): a Brownian motion on ''M'' is defined to be a diffusion on ''M'' whose characteristic operator in local coordinates ''x''''i'', 1 ≤ ''i'' ≤ ''m'', is given by ½ΔLB, where ΔLB is the Laplace–Beltrami operator given in local coordinates by
:
where [''g''''ij''] = [''g''''ij'']−1 in the sense of Invertible matrix, the inverse of a square matrix.
Narrow escape
The narrow escape problem is a ubiquitous problem in biology, biophysics and cellular biology which has the following formulation: a Brownian particle (ion,molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
, or protein) is confined to a bounded domain (a compartment or a cell) by a reflecting boundary, except for a small window through which it can escape. The narrow escape problem is that of calculating the mean escape time. This time diverges as the window shrinks, thus rendering the calculation a singular perturbation problem.
See also
* Brownian bridge: a Brownian motion that is required to "bridge" specified values at specified times * Brownian covariance * Brownian dynamics * Brownian motion of sol particles * Brownian motor * Brownian noise (Martin Gardner proposed this name for sound generated with random intervals. It is a pun on Brownian motion and white noise.) * Brownian ratchet * Brownian surface * Brownian tree * Brownian web * Rotational Brownian motion * Free will in antiquity#Epicureanism, Clinamen * Complex system * Continuity equation * Diffusion equation * Geometric Brownian motion * Itô diffusion: a generalisation of Brownian motion * Langevin equation * Lévy arcsine law * Local time (mathematics) * Many-body problem * Marangoni effect * Nanoparticle tracking analysis * Narrow escape problem * Osmosis * Random walk * Schramm–Loewner evolution * Single particle trajectories * Single particle tracking * Statistical mechanics * Surface diffusion: a type of constrained Brownian motion. * Thermal equilibrium * Thermodynamic equilibrium * Triangulation sensing * Tyndall effect: a phenomenon where particles are involved; used to differentiate between the different types of mixtures. * UltramicroscopeReferences
Further reading
* Also includes a subsequent defense by Brown of his original observations, ''Additional remarks on active molecules''. * * * * * * *Lucretius
Titus Lucretius Carus ( , ; – ) was a Roman poet and philosopher. His only known work is the philosophical poem ''De rerum natura'', a didactic work about the tenets and philosophy of Epicureanism, and which usually is translated into En ...
, ''On The Nature of Things'', translated by William Ellery Leonard. (on-line version
', from Project Gutenberg. See the heading 'Atomic Motions'; this translation differs slightly from the one quoted). * Edward Nelson, Nelson, Edward, (1967). ''Dynamical Theories of Brownian Motion''
(PDF version of this out-of-print book, from the author's webpage.)
This is primarily a mathematical work, but the first four chapters discuss the history of the topic, in the era from Brown to Einstein. * * ** See also Perrin's book "Les Atomes" (1914). * * * Thorvald N. Thiele, Theile, T. N. ** Danish version: "Om Anvendelse af mindste Kvadraters Methode i nogle Tilfælde, hvor en Komplikation af visse Slags uensartede tilfældige Fejlkilder giver Fejlene en 'systematisk' Karakter". ** French version: "Sur la compensation de quelques erreurs quasi-systématiques par la méthodes de moindre carrés" published simultaneously in ''Vidensk. Selsk. Skr. 5. Rk., naturvid. og mat. Afd.'', 12:381–408, 1880.
External links
Einstein on Brownian Motion
* [http://www.gizmag.com/einsteins-prediction-finally-witnessed/16212/ "Einstein's prediction finally witnessed one century later"] : a test to observe the velocity of Brownian motion
Large-Scale Brownian Motion Demonstration
{{Authority control Statistical mechanics Wiener process Fractals Colloidal chemistry Robert Brown (botanist, born 1773) Albert Einstein Articles containing video clips Lévy processes