Borophene on:
[Wikipedia]
[Google]
[Amazon]
 Borophene is a
Borophene is a
 Computational studies by I. Boustani and A. Quandt showed that small boron clusters do not adopt icosahedral geometries like
Computational studies by I. Boustani and A. Quandt showed that small boron clusters do not adopt icosahedral geometries like
 Borophene is a
Borophene is a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
line atomic monolayer of boron, i.e., it is a two-dimensional
In mathematics, a plane is a Euclidean ( flat), two-dimensional surface that extends indefinitely. A plane is the two-dimensional analogue of a point (zero dimensions), a line (one dimension) and three-dimensional space. Planes can arise as ...
allotrope of boron and also known as ''boron sheet''.
First predicted by theory in the mid-1990s,
different borophene structures were experimentally confirmed in 2015.
Properties
Experimentally various atomically thin,crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
line and metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
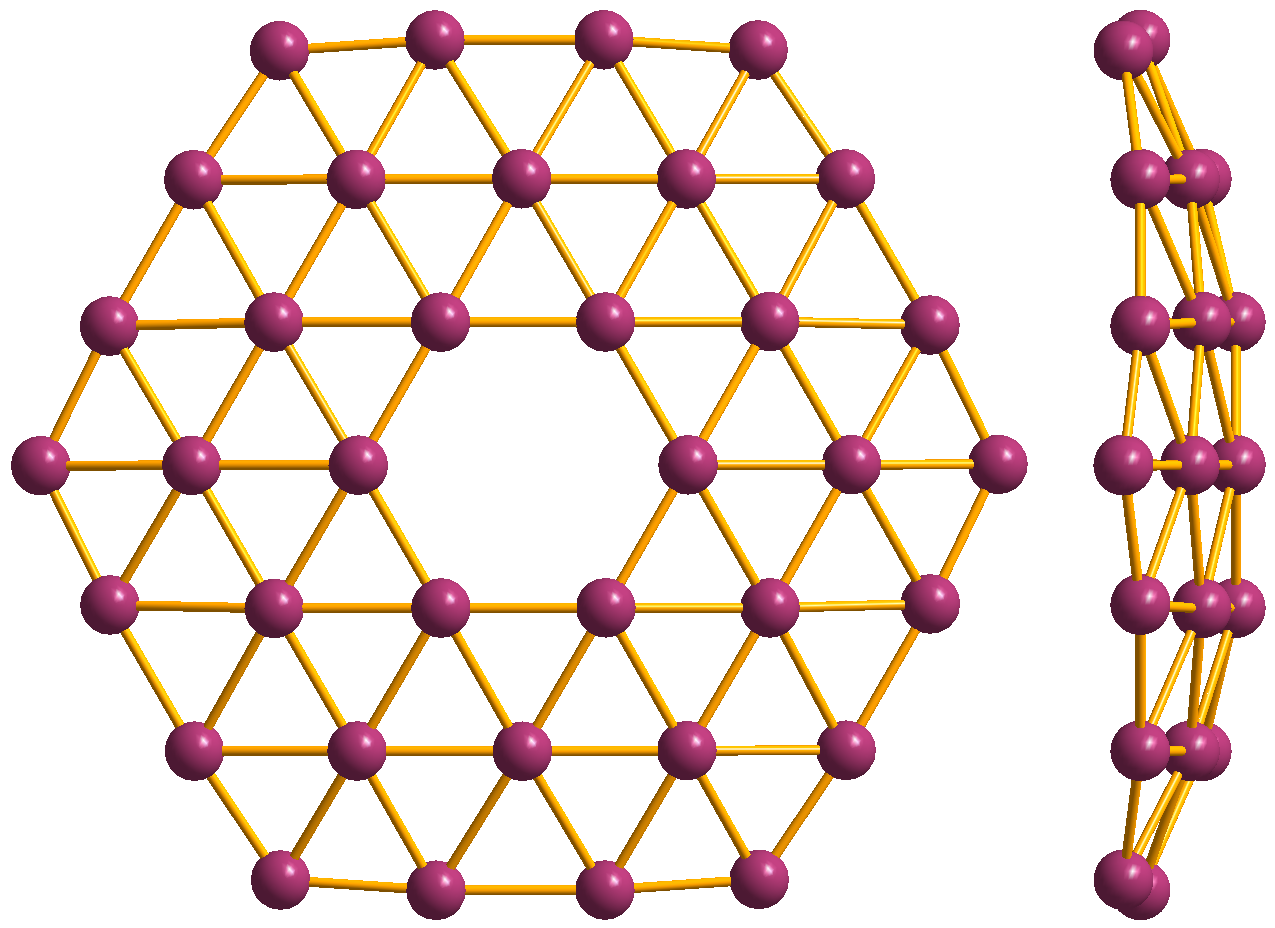
lic borophenes were synthesized on clean metal surfaces under ultrahigh-vacuum conditions. Its atomic structure consists of mixed triangular and hexagonal motifs, such as shown in Figure 1. The atomic structure is a consequence of an interplay between two-center and multi-center in-plane bonding, which is typical for electron deficient elements like boron.
Borophenes exhibit in-plane elasticity and ideal strength. It can be stronger than graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
, and more flexible, in some configurations. Boron nanotubes are also stiffer than graphene, with a higher 2D Young's modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied le ...
than any other known carbon and noncarbon nanostructures. Borophenes undergo novel structural phase transition under in-plane tensile loading due to the fluxional nature of their multi-center in-plane bonding. Borophene has potential as an anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic ...
material for batteries due to high theoretical specific capacities, electronic conductivity, and ion transport properties. Hydrogen easily adsorbs to borophene, offers potential for hydrogen storage – over 15% of its weight. Borophene can catalyze the breakdown of molecular hydrogen into hydrogen ions, and reduce water.
History
 Computational studies by I. Boustani and A. Quandt showed that small boron clusters do not adopt icosahedral geometries like
Computational studies by I. Boustani and A. Quandt showed that small boron clusters do not adopt icosahedral geometries like boranes
Boranes is the name given to compounds with the formula BxHy and related anions. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bond ...
, instead they turn out to be quasi-planar (see Figure 2). This led to the discovery of a so-called Aufbau principle that predicts the possibility of borophene (boron sheets), boron fullerenes (borospherene
Borospherene (B40) is a cluster molecule containing 40 boron atoms. It is similar to buckminsterfullerene, the "spherical" carbon structure, but with a different symmetry. The discovery of borospherene was announced in July 2014, and is described ...
) and boron nanotubes.
Additional studies showed that extended, triangular borophene (Figure 1(c)) is metallic and adopts a non-planar, buckled geometry. Further computational studies, initiated by the prediction of a stable B80 boron fullerene, suggested that extended borophene sheets with honeycomb structure and with partially filled hexagonal holes are stable. These borophene structures were predicted to be metallic. The so-called γ sheet (a.k.a. β12 borophene or υ1/6 sheet) is shown in Figure 1(a).
The planarity of boron clusters was first experimentally confirmed by the research team of L.-S. Wang. Later they showed that the structure of (see Figure 2) is the smallest boron cluster to have sixfold symmetry and a perfect hexagonal vacancy, and that it can serve as a potential basis for extended two-dimensional boron sheets.
After the synthesis of silicene, multiple groups predicted that borophene could potentially be realized with the support of a metal surface. In particular, the lattice structure of borophene was shown to depend on the metal surface, displaying a disconnect from that in a freestanding state.
In 2015 two research teams succeeded in synthesizing different borophene phases on silver (111) surfaces under ultrahigh-vacuum conditions. Among the three borophene phases synthesized (see Figure 1), the v1/6 sheet, or ''β''12, was shown by an earlier theory to be the ground state on the Ag(111) surface, while the ''χ''3 borophene was previously predicted by Zeng team in 2012. So far, borophenes exist only on substrates; how to transfer them onto a device-compatible substrate is necessary, but remains a challenge.
Atomic-scale characterization, supported by theoretical calculations, revealed structures reminiscent of fused boron clusters consisting of mixed triangular and hexagonal motives, as previously predicted by theory and shown in Figure 1. Scanning tunneling spectroscopy
Scanning tunneling spectroscopy (STS), an extension of scanning tunneling microscopy (STM), is used to provide information about the density of electrons in a sample as a function of their energy.
In scanning tunneling microscopy, a metal tip i ...
confirmed that the borophenes are metallic. This is in contrast to bulk boron allotropes, which are semiconducting and marked by an atomic structure based on B12 icosahedra.
In 2021 researchers announced hydrogenated
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
borophene on a silver substrated, dubbed borophane. The new material was claimed to be far more stable than its component. Hydrogenation reduces oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
rates by more than two orders of magnitude after ambient exposure. The creation of two-layer borophene was announced in August 2021.Liu, Xiaolong; Li, Qiucheng; Ruan, Qiyuan; Rahn, Matthew S.; Yakobson, Boris I.; Hersam, Mark C. "Borophene synthesis beyond the single-atomic-layer limit." ''Nature Materials'' (26 August 2021). https://doi.org/10.1038/s41563-021-01084-2
See also
*Allotropes of boron
Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of ...
*Borospherene
Borospherene (B40) is a cluster molecule containing 40 boron atoms. It is similar to buckminsterfullerene, the "spherical" carbon structure, but with a different symmetry. The discovery of borospherene was announced in July 2014, and is described ...
References
External links
*{{Commons category-inline Allotropes of boron Monolayers Substances discovered in the 2010s