Blanc chloromethylation on:
[Wikipedia]
[Google]
[Amazon]
The Blanc chloromethylation (also called the Blanc reaction) is the 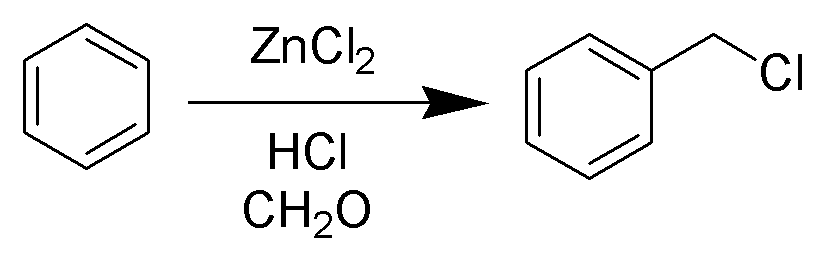
 Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates that are inert to Friedel-Crafts reactions like acetophenone, nitrobenzene and ''p''-chloronitrobenzene do show marginal reactivity of limited synthetic utility under chloromethylation conditions. Deactivated substrates give better results under modified chloromethylation conditions using chloromethyl methyl ether (MOMCl) in the presence of 60% H2SO4.
Highly activated arenes like phenols and anilines are not suitable substrates, since they undergo further electrophilic attack by Friedel-Crafts alkylation with the formed benzylic alcohol/chloride in an uncontrolled manner. In general, the formation of diarylmethane side product is a common outcome.
Although the reaction is an efficient means of introducing a chloromethyl group, the production of small amounts of highly carcinogenic bis(chloromethyl) ether is a disadvantage for industrial applications.
The corresponding fluoromethylation, bromomethylation and iodomethylation reactions can also be achieved, using the appropriate hydrohalic acid.
Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates that are inert to Friedel-Crafts reactions like acetophenone, nitrobenzene and ''p''-chloronitrobenzene do show marginal reactivity of limited synthetic utility under chloromethylation conditions. Deactivated substrates give better results under modified chloromethylation conditions using chloromethyl methyl ether (MOMCl) in the presence of 60% H2SO4.
Highly activated arenes like phenols and anilines are not suitable substrates, since they undergo further electrophilic attack by Friedel-Crafts alkylation with the formed benzylic alcohol/chloride in an uncontrolled manner. In general, the formation of diarylmethane side product is a common outcome.
Although the reaction is an efficient means of introducing a chloromethyl group, the production of small amounts of highly carcinogenic bis(chloromethyl) ether is a disadvantage for industrial applications.
The corresponding fluoromethylation, bromomethylation and iodomethylation reactions can also be achieved, using the appropriate hydrohalic acid.
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
of aromatic rings with formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
and hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic and e ...
. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923

Mechanism and scope
The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions. Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates that are inert to Friedel-Crafts reactions like acetophenone, nitrobenzene and ''p''-chloronitrobenzene do show marginal reactivity of limited synthetic utility under chloromethylation conditions. Deactivated substrates give better results under modified chloromethylation conditions using chloromethyl methyl ether (MOMCl) in the presence of 60% H2SO4.
Highly activated arenes like phenols and anilines are not suitable substrates, since they undergo further electrophilic attack by Friedel-Crafts alkylation with the formed benzylic alcohol/chloride in an uncontrolled manner. In general, the formation of diarylmethane side product is a common outcome.
Although the reaction is an efficient means of introducing a chloromethyl group, the production of small amounts of highly carcinogenic bis(chloromethyl) ether is a disadvantage for industrial applications.
The corresponding fluoromethylation, bromomethylation and iodomethylation reactions can also be achieved, using the appropriate hydrohalic acid.
Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrates that are inert to Friedel-Crafts reactions like acetophenone, nitrobenzene and ''p''-chloronitrobenzene do show marginal reactivity of limited synthetic utility under chloromethylation conditions. Deactivated substrates give better results under modified chloromethylation conditions using chloromethyl methyl ether (MOMCl) in the presence of 60% H2SO4.
Highly activated arenes like phenols and anilines are not suitable substrates, since they undergo further electrophilic attack by Friedel-Crafts alkylation with the formed benzylic alcohol/chloride in an uncontrolled manner. In general, the formation of diarylmethane side product is a common outcome.
Although the reaction is an efficient means of introducing a chloromethyl group, the production of small amounts of highly carcinogenic bis(chloromethyl) ether is a disadvantage for industrial applications.
The corresponding fluoromethylation, bromomethylation and iodomethylation reactions can also be achieved, using the appropriate hydrohalic acid.
Related chloromethylations
Chloromethylation of thiols can be effected with concentrated HCl and formaldehyde: :ArSH + CH2O + HCl → ArSCH2Cl + H2O Chloromethylation can also be effected using chloromethyl methyl ether: :ArH + CH3OCH2Cl → ArCH2Cl + CH3OH This reaction is employed in the chloromethylation of styrene in the production of ion-exchange resins and Merrifield resins.Additional reading
*Safety
The reaction is performed with care as, like most chloromethylation reactions, it produces highlycarcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive subs ...
ic bis(chloromethyl) ether as a by-product.
See also
* Friedel-Crafts alkylation * Quelet reaction *Bouveault–Blanc reduction
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demon ...
References
{{Organic reactions Carbon-carbon bond forming reactions Name reactions Substitution reactions