Bcr-Abl tyrosine-kinase inhibitor on:
[Wikipedia]
[Google]
[Amazon]
Bcr-Abl tyrosine-kinase inhibitors (TKI) are the first-line therapy for most patients with
 In the development of imatinib, the
In the development of imatinib, the
 Since then crystallographic studies have revealed that imatinib binds to the kinase
Since then crystallographic studies have revealed that imatinib binds to the kinase
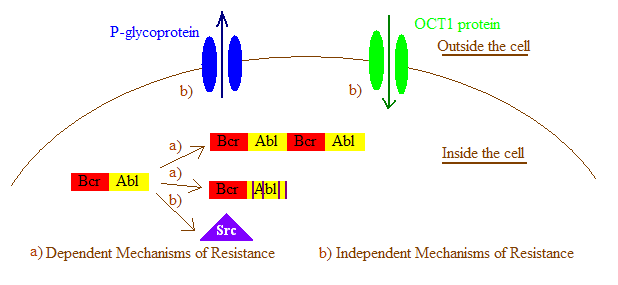 In general, imatinib resistance can be subdivided into Bcr-Abl dependent and independent mechanisms. Bcr-Abl dependent mechanisms include over expression or amplification of the Bcr-Abl gene and
In general, imatinib resistance can be subdivided into Bcr-Abl dependent and independent mechanisms. Bcr-Abl dependent mechanisms include over expression or amplification of the Bcr-Abl gene and




 Bosutinib's structure is based on a
Bosutinib's structure is based on a
 Ariad used the highly potent druglead, AP23464 to further investigate inhibitory possibilities of purine cored templates for dual Src/Abl inhibitors. First, searching for substances effective on the inactive conformation of Abl, the side chain bound to the nitrogen on the purine core was replaced with a di
Ariad used the highly potent druglead, AP23464 to further investigate inhibitory possibilities of purine cored templates for dual Src/Abl inhibitors. First, searching for substances effective on the inactive conformation of Abl, the side chain bound to the nitrogen on the purine core was replaced with a di
 Due to the structural similarities of imatinib and bafetinib, their binding to Bcr-Abl is also quite similar. The only notable difference comes from the hydrophobic interaction between the trifluoromethyl group and the hydrophobic pocket created by Ile-293, Leu-298, Leu-354, and Val-379. This group can also be linked to bafetinib's specificity for Lyn, as the binding site there is almost identical to that on Bcr-Abl.
Bafetinib has its place in TKI therapy as it is effective both against most imatinib resistant mutations (not including T315I) and some dasatinib resistant mutations. Bafetinib also has more affinity for Bcr-Abl than nilotinib (but less than dasatinib) but only targets Bcr-Abl and Src family kinases Lck and Lyn; with unrivalled specificity which suggests the probability of fewer adverse effects.
CytRx has bafetinb in phase II clinical trial as a treatment for leukemia as of May 2010.
Due to the structural similarities of imatinib and bafetinib, their binding to Bcr-Abl is also quite similar. The only notable difference comes from the hydrophobic interaction between the trifluoromethyl group and the hydrophobic pocket created by Ile-293, Leu-298, Leu-354, and Val-379. This group can also be linked to bafetinib's specificity for Lyn, as the binding site there is almost identical to that on Bcr-Abl.
Bafetinib has its place in TKI therapy as it is effective both against most imatinib resistant mutations (not including T315I) and some dasatinib resistant mutations. Bafetinib also has more affinity for Bcr-Abl than nilotinib (but less than dasatinib) but only targets Bcr-Abl and Src family kinases Lck and Lyn; with unrivalled specificity which suggests the probability of fewer adverse effects.
CytRx has bafetinb in phase II clinical trial as a treatment for leukemia as of May 2010.
 One Italian research group discovered through digital screening that commercially available thiadiazole derivatives displayed moderate inhibitory action on both Abl and Src kinases. Using a 1,3,4 thiadiazol core and trying different groups or molecules on the benzene rings, several different substances with inhibitory properties were produced. The flexibility of the core allowed a number of conformations of the substances to bind to the ATP site of the Abl kinase, though all of them bound to the kinase's active form. Further study of the binding showed that the position of the sulfur that binds to the toluene structure played an important role in regard to Abl binding and also that only one of the nitrogen's one thiadiazole formed a hydrogen bond. Furthermore, computer analysis of the structure showed the amide connected benzene-ketone could be substituted for a more favorable
One Italian research group discovered through digital screening that commercially available thiadiazole derivatives displayed moderate inhibitory action on both Abl and Src kinases. Using a 1,3,4 thiadiazol core and trying different groups or molecules on the benzene rings, several different substances with inhibitory properties were produced. The flexibility of the core allowed a number of conformations of the substances to bind to the ATP site of the Abl kinase, though all of them bound to the kinase's active form. Further study of the binding showed that the position of the sulfur that binds to the toluene structure played an important role in regard to Abl binding and also that only one of the nitrogen's one thiadiazole formed a hydrogen bond. Furthermore, computer analysis of the structure showed the amide connected benzene-ketone could be substituted for a more favorable
chronic myelogenous leukemia
Chronic myelogenous leukemia (CML), also known as chronic myeloid leukemia, is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumul ...
(CML). More than 90% of CML cases are caused by a chromosomal abnormality that results in the formation of a so-called Philadelphia chromosome
The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells (particularly chronic myeloid leukemia (CML) cells). This chromosome is defective and unusually short becaus ...
. This abnormality was discovered by Peter Nowell in 1960 and is a consequence of fusion between the Abelson ( Abl) tyrosine kinase gene at chromosome 9
Chromosome 9 is one of the 23 pairs of chromosomes in humans. Humans normally have two copies of this chromosome, as they normally do with all chromosomes. Chromosome 9 spans about 138 million base pairs of nucleic acids (the building blocks of D ...
and the break point cluster ( Bcr) gene at chromosome 22
Chromosome 22 is one of the 23 pairs of chromosomes in human cells. Humans normally have two copies of chromosome 22 in each cell. Chromosome 22 is the second smallest human chromosome, spanning about 49 million DNA base pairs and representing ...
, resulting in a chimeric oncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.
(Bcr-Abl
The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells (particularly chronic myeloid leukemia (CML) cells). This chromosome is defective and unusually short becaus ...
) and a constitutively active Bcr-Abl tyrosine kinase that has been implicated in the pathogenesis
Pathogenesis is the process by which a disease or disorder develops. It can include factors which contribute not only to the onset of the disease or disorder, but also to its progression and maintenance. The word comes from Greek πάθος ''pat ...
of CML. Compounds have been developed to selectively inhibit the tyrosine kinase.
Before the 2001 U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
(FDA) approval of imatinib
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral chemotherapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple receptor tyrosine kin ...
, no drugs were available to alter the natural progression of CML. Only cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating c ...
drugs such as busulfan
Busulfan (Myleran, GlaxoSmithKline, Busulfex IV, Otsuka America Pharmaceutical, Inc.) is a chemotherapy drug in use since 1959. It is a cell cycle non-specific alkylating antineoplastic agent, in the class of alkyl sulfonates. Its chemical ...
, hydroxyurea
Hydroxycarbamide, also known as hydroxyurea, is a medication used in sickle-cell disease, essential thrombocythemia, chronic myelogenous leukemia, polycythemia vera, and cervical cancer. In sickle-cell disease it increases fetal hemoglobin and d ...
or interferon
Interferons (IFNs, ) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten th ...
-alpha (rIFN-α) were utilized. Even though the first Bcr-Abl TK inhibitor was named "the magic bullet" to cure cancer by ''Time
Time is the continued sequence of existence and event (philosophy), events that occurs in an apparently irreversible process, irreversible succession from the past, through the present, into the future. It is a component quantity of various me ...
'' magazine, a second generation of Bcr-Abl TKI was subsequently developed to combat the initial resistance that emerged.
New forms of resistance can arise as: missense mutations
In genetics, a missense mutation is a point mutation in which a single nucleotide change results in a codon that codes for a different amino acid. It is a type of nonsynonymous substitution.
Substitution of protein from DNA mutations
Missense m ...
within the Abl kinase domain
Domain may refer to:
Mathematics
*Domain of a function, the set of input values for which the (total) function is defined
** Domain of definition of a partial function
** Natural domain of a partial function
**Domain of holomorphy of a function
* ...
, over-expression of Bcr-Abl, increased production of transmembrane plasma protein
Blood-proteins, also termed plasma proteins, are proteins present in blood plasma. They serve many different functions, including transport of lipids, hormones, vitamins and minerals in activity and functioning of the immune system. Other bl ...
s, or the constitutive activation of downstream signaling molecules such as Src-family kinases.
History
CML has a well defined molecular target and relatively selective therapies aimed at that target, which is not the case for the majority of cancers andchemotherapies
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemothera ...
today. Bcr-Abl was regarded as highly attractive target for drug intervention since the Bcr-Abl fusion gene A fusion gene is a hybrid gene formed from two previously independent genes. It can occur as a result of translocation, interstitial deletion, or chromosomal inversion. Fusion genes have been found to be prevalent in all main types of human neopla ...
encodes a constitutively activated kinase. Drug discovery that specifically targeted the ATP binding site of a single kinase was regarded as quite a challenging task since hundreds of protein kinases were known in the human genome
The human genome is a complete set of nucleic acid sequences for humans, encoded as DNA within the 23 chromosome pairs in cell nuclei and in a small DNA molecule found within individual mitochondria. These are usually treated separately as the ...
. In the presence of TKI the binding of ATP is blocked, phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
is prevented and Bcr-Abl expressing cells either have a selective growth disadvantage or undergo apoptotic
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes incl ...
cell death.
Due to increasing resistance and intolerance to imatinib efforts were made to develop new drugs that could inhibit the Bcr-Abl tyrosine kinase. This led to the discovery of second generation drugs. While drug screening was used to develop imatinib, second generation TKI's were developed with rational drug design
Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activa ...
approach due to increased knowledge in structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
of the Bcr-Abl tyrosine kinase.
First generation
Imatinib (STI571)
Imatinib
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral chemotherapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple receptor tyrosine kin ...
(Gleevec) was discovered in 1992 and is regarded as first generation drug since it is the first Bcr-Abl tyrosine kinase inhibitor to be used in the treatment of CML.
Development
structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such a ...
of Bcr-Abl tyrosine kinase played a limited role because it was unknown. A high-throughput screening
High-throughput screening (HTS) is a method for scientific experimentation especially used in drug discovery and relevant to the fields of biology, materials science and chemistry. Using robotics, data processing/control software, liquid handling ...
of chemical libraries at Novartis
Novartis AG is a Swiss-American multinational pharmaceutical corporation based in Basel, Switzerland and
Cambridge, Massachusetts, United States (global research).name="novartis.com">https://www.novartis.com/research-development/research-lo ...
was performed to identify a starting molecule, which was called "Pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The othe ...
A". This compound served as a lead compound
A lead compound (, i.e. a "leading" compound, not to be confused with various compounds of the metallic element lead) in drug discovery is a chemical compound that has pharmacology, pharmacological or biological activity likely to be therapeutical ...
and was then tested and modified to develop imatinib. With a replacement of the imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...
group with a benzamido group, the compound's specificity increased while its activity as a kinase inhibitor remained the same. Subsequently, introducing a methyl subtituent ortho to the pyrimidinyl-amino group enhanced the potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
.
Binding
domain
Domain may refer to:
Mathematics
*Domain of a function, the set of input values for which the (total) function is defined
** Domain of definition of a partial function
** Natural domain of a partial function
**Domain of holomorphy of a function
* ...
of Abl only when the domain adopts the inactive or "closed" conformation.
This is where the glycine-rich, P-binding phosphate loop (P-loop) folds over the ATP binding site and the activation-loop adopts a conformation in which it occludes the substrate binding site and disrupts the ATP phosphate binding site to block the catalytic activity of the enzyme. The shift of the Asp Phe Gly (DFG) triad at the N-terminal end of the activation loop
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs ran ...
results in the exposure of a binding pocket which can be utilized by inhibitors. This conformation is referred to as DFGout.
Imatinib binds to Abl domain via six hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
interactions. This stabilizes the imatinib
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral chemotherapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple receptor tyrosine kin ...
Bcr-Abl complex and prevents ATP from reaching its binding site. The hydrogen bonds involve the pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
-N and backbone
The backbone is the vertebral column of a vertebrate.
Arts, entertainment, and media Film
* ''Backbone'' (1923 film), a 1923 lost silent film starring Alfred Lunt
* ''Backbone'' (1975 film), a 1975 Yugoslavian drama directed by Vlatko Gilić
...
-NH of Met-318, the aminopyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The othe ...
and side chain hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
of Thr-315, the amide-NH and side chain carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
ate of Glu-286, the carbonyl and backbone-NH of Asp-381, the protonated methylpiperazine
Piperazine () is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
The piperazines are a broad ...
with the backbone-carbonyl atoms of Ile
Ile may refer to:
* iLe, a Puerto Rican singer
* Ile District (disambiguation), multiple places
* Ilé-Ifẹ̀, an ancient Yoruba city in south-western Nigeria
* Interlingue (ISO 639:ile), a planned language
* Isoleucine, an amino acid
* Another ...
-360 and His-361. Additionally, a number of van der Waals interactions contribute to binding. A hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
pocket is formed by amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
residues Ile-293, Leu
Leu may refer to:
Businesses and organisations
* LEU, NYSE American stock symbol for Centrus Energy Corp.
* London Ecology Unit, a former body (1986-2000) which advised London boroughs on environmental matters
* Free and Equal (''LeU - Liberi e ...
-298, Leu-354 and Val
Val may refer to: Val-a
Film
* ''Val'' (film), an American documentary about Val Kilmer, directed by Leo Scott and Ting Poo
Military equipment
* Aichi D3A, a Japanese World War II dive bomber codenamed "Val" by the Allies
* AS Val, a Sov ...
-379 around the phenyl ring adjacent to the piperazinyl-methyl group of imatinib. At the time of its discovery, in the absence of structural information, no clear explanation for the impressive selectivity of imatinib could be found.
Although first-generation treatment achieved an extremely high response rate and a low relapse rate in CML patients, some patients do experience resistance or intolerance to imatinib.
Drug resistance
Drug resistance
Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is ...
is the main drive in continuing research and development of Bcr-Abl TKI. Shortly after the introduction of imatinib, investigators began to describe a number of in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology a ...
derived cell lines
An immortalised cell line is a population of cells from a multicellular organism which would normally not proliferate indefinitely but, due to mutation, have evaded normal cellular senescence and instead can keep undergoing division. The cells ...
with resistance to the drug. This was rapidly followed by the clinical description of imatinib resistant cells in patients, which has resulted in efforts to better understand the biology behind these observations. Assessments of therapeutic response of imatinib in patients with CML are based upon meeting hematologic, cytogenic and molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
milestones. Patients that fail to achieve defined responses at predefined time points are described as primarily resistant to therapy, and those losing previously obtained milestones in disease regression are termed secondarily resistant. Before a conclusion is drawn, it is important to consider that retrospective
A retrospective (from Latin ''retrospectare'', "look back"), generally, is a look back at events that took place, or works that were produced, in the past. As a noun, ''retrospective'' has specific meanings in medicine, software development, popu ...
data has showed a high incidence of imatinib non-compliance
Compliance can mean:
Healthcare
* Compliance (medicine), a patient's (or doctor's) adherence to a recommended course of treatment
* Compliance (physiology), the tendency of a hollow organ to resist recoil toward its original dimensions (this is a ...
in CML patients and this could lead to undesired clinical outcomes.
point mutations
A point mutation is a genetic mutation where a single nucleotide base is changed, inserted or deleted from a DNA or RNA sequence of an organism's genome. Point mutations have a variety of effects on the downstream protein product—consequence ...
within the Bcr-Abl kinase domain that interfere with imatinib binding. Bcr-Abl independent mechanisms include factors influencing the concentration of imatinib within the cell, for example by alterations in drug influx and efflux and activation of Bcr-Abl independent pathways, such as members of the Src kinase family. Imatinib resistance can also be produced by other mechanisms that will not be mentioned here as the importance of those mechanisms still remain a question due to lack of clinical data.
Bcr-Abl dependent mechanisms of resistance
Bcr-Abl duplication
The first reports of resistance to imatinib described a development of oncogene amplification. That is, thegene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
that encodes for the pathogenic Bcr-Abl tyrosine kinase is duplicated in the DNA sequence
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, guanine, cytosine, and thymine. T ...
, leading to higher expression of the pathogen. Increasing the imatinib dose could surmount this kind of resistance, provided that severe or intolerable adverse effects
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complica ...
are not produced.
Bcr-Abl mutation
Point mutations
A point mutation is a genetic mutation where a single nucleotide base is changed, inserted or deleted from a DNA or RNA sequence of an organism's genome. Point mutations have a variety of effects on the downstream protein product—consequence ...
can cause amino acid substitutions inside the kinase domain of the Bcr-Abl protein and disrupt the binding site of imatinib on the tyrosine kinase, resulting in a loss of sensitivity to the drug. These mutations normally affect the structure of the Bcr-Abl protein, leading either to interruption of critical contact points between the drug and the Bcr-Abl protein or induction of a conformational change, resulting in a protein that imatinib is unable to bind to.
Mutational frequencies appear to increase as the disease, CML, progresses from chronic phase to the blast phase. The most important mutations are the P-loop
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982.
Of the two motifs, the A motif is t ...
mutations and the T315I mutation. Mutations on other sites of the kinase have also been reported, for example on the C-helix
A helix () is a shape like a corkscrew or spiral staircase. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined helic ...
, SH2 domain
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains allow proteins containing those domains to dock to phosphor ...
, substrate binding site, activation loop and C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
lobe. Some of these mutations have clinical significance, but none as much as P-loop
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982.
Of the two motifs, the A motif is t ...
and T315I mutations.
=T315I mutation
= The T315I is a unique mutation because of its resistance to all approved Bcr-Abl inhibitors, prior toponatinib
Ponatinib (trade name Iclusig , previously AP24534) is an oral drug developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a ...
. It is caused by a single cytosine
Cytosine () ( symbol C or Cyt) is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached ( ...
to thymine
Thymine () ( symbol T or Thy) is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidin ...
(C -> T) base pair
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both D ...
substitution at position 944 of the Abl gene (codon
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
'315' of the Abl protein) sequence resulting in amino acid (T)hreonine being substituted by (I)soleucine at that position - thus 'T315I'. This substitution eliminates a critical oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
molecule needed for hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
ing between imatinib and the Abl kinase, and also creates steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
to the binding of most TKIs.
When discovered, it was estimated that every 6 out of 9 cases of advanced stage CML with imatinib resistance carried this mutation. T315I produces the highest magnitude of resistance of any mutation both to imatinib and second generations TKIs. Ponatinib
Ponatinib (trade name Iclusig , previously AP24534) is an oral drug developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a ...
(Iclusig) by Ariad was approved in 2013 for use as second-line CML treatment, and is the only licensed TKI which binds to the T315I mutated kinase successfully.
=P-loop mutations
= The structure of Bcr-Abl contains two flexible loops, the ATP-bindingP-loop
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982.
Of the two motifs, the A motif is t ...
and the activation loop. These loops have specific arrangements in the inactive conformation of Bcr-Abl that stabilize the basal conformation. Mutations in these loops destabilize arrangement of the loops such that the kinase domain cannot assume the inactive conformation required for imatinib binding. Mutations in the P-loop region are the most common, accounting for 36-48% of all mutations. There are clinical data indicating that Bcr-Abl mutations in the P-loop is 70-100 fold less sensitive to imatinib compared with native Bcr-Abl.
Bcr-Abl Independent mechanisms of resistance
Additional mechanisms have been postulated to describe resistance seen in various model systems although none have been clearly identified as a sole source of clinical resistance.Drug efflux caused by P-glycoproteins
Some investigations in cell lines have shown that imatinib resistance may be partly due to an increase in the expression of theP-glycoprotein
P-glycoprotein 1 (permeability glycoprotein, abbreviated as P-gp or Pgp) also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1) or cluster of differentiation 243 (CD243) is an important protein ...
efflux pump. By utilizing agents that inhibit P-glycoprotein activity imatinib susceptibility has been restored in some cases.
Drug import by organic cation transporter 1
The entry of imatinib into cells is dependent on an organic cation transporter ( OCT1). OCT1 plays a significant role in imatinib resistance by inhibiting its influx and thus decreasing the intracellular bioavailability of imatinib. Patients with low expression, activity or polymorphisms of OCT1 had significantly lower intracellular levels of imatinib. The response of patients with low OCT1 activity was significantly dose-dependent. This data indicates that OCT1 activity is an important determinant in the molecular response to imatinib.Alternative signaling pathway activation
In a few patient groups, resistance may be caused by the activation of other signaling pathways, particularly the Src family kinases. The Src family kinases have been implicated in Bcr-Abl signaling and mediate imatinib resistance by stabilizing the active conformation of Bcr-Abl, a conformation that does not bind imatinib. Furthermore, increasing evidence suggests that Src family kinases are also involved in Bcr-Abl-independent forms of imatinib resistance.Solutions
The treatment options for imatinib resistant or intolerant CML patients may include strategies such as increasing the dose of imatinib or the use of second-generation drugs. Escalation of imatinib-doses has shown to overcome some cases of primary resistance to imatinib, such as Bcr-Abl duplication, but the response is usually short acting. In the case of resistance or intolerance, it could be helpful to test for Bcr-Abl mutations to direct the choice of second line treatment as the variable options have different function profile against the different mechanisms of resistance. Second-generation drugs offer improvedpotency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
and a greater likelihood of success in resistant patients. There is also a growing interest in testing the hypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous obse ...
that administration of multiple Abl kinase inhibitors in early phase patients could be used to delay or prevent the emergence of drug resistant clones
Clone or Clones or Cloning or Cloned or The Clone may refer to:
Places
* Clones, County Fermanagh
* Clones, County Monaghan, a town in Ireland
Biology
* Clone (B-cell), a lymphocyte clone, the massive presence of which may indicate a pathologi ...
. The combination of two agents targeting different pathways involved in CML may significantly improve response rates and potentially increase survival.
Second generation drugs
Second generation drugs are intended to have decreased resistance and intolerance than imatinib. Second generation drugs that are currently marketed are nilotinib, dasatinib, bosutinib and ponatinib.Nilotinib (AMN107)
Development
Nilotinib
Nilotinib, sold under the brand name Tasigna marketed worldwide by Novartis, is a medication used to treat chronic myelogenous leukemia (CML) which has the Philadelphia chromosome. It may be used both in initial cases of chronic phase CML as well ...
is a phenylamino-pyrimidine derivative that is structurally related to imatinib. It was developed based on the structure of the Abl-imatinib complex to address the need associated with imatinib intolerance and resistance. Small changes were made on the imatinib molecule to make it more potent and selective as a Bcr-Abl inhibitor and these changes resulted in the discovery of nilotinib. Nilotinib is a selective Bcr-Abl kinase inhibitor.
Nilotinib is 10-30 fold more potent than imatinib in inhibiting activity of the Bcr-Abl tyrosine kinase and proliferation of Bcr-Abl expressing cells. The drug effectively inhibits the auto phosphorylation of Bcr-Abl on Tyr-177 that is involved in CML pathogenesis. Synergistic
Synergy is an interaction or cooperation giving rise to a whole that is greater than the simple sum of its parts. The term ''synergy'' comes from the Attic Greek word συνεργία ' from ', , meaning "working together".
History
In Christia ...
activity of imatinib and nilotinib has been reported following coadministration. This might be a result of the fact that the drugs are taken up in cells by different mechanisms: imatinib influx is dependent on OCT1 but nilotinib is not. Nilotinib is also not a substrate for the efflux transporter P-glycoprotein pump, unlike imatinib. Although the two dimensional molecular structures of these two drugs might look similar, they are dissimilar in terms of spatial structure and molecular properties.

Binding
Nilotinib binds to the inactive conformation of the Abl kinase domain, largely throughlipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipo ...
interactions and thus blocks its catalytic activity. Nilotinib binds to the kinase domain by making four hydrogen bond interactions involving the pyridyl-N and the backbone NH of Met-318, the anilino-NH and the side chain OH of Thr-315, the amido-NH and side chain carboxylate of Glu-286 and the amido carbonyl with the backbone NH of the Asp-381. The -(3-pyridinyl)-2-pyrimidinylanilino segment of nilotinib has close binding interactions with Met-318, Phe-317 and Thr-315 residues of a region within the ATP binding site. The remaining half of the compound extends beyond the Thr-315 gatekeeper residue to bind within an additional pocket. The 3-methylimidazole and trifluoro-methyl groups of nilotinib make important interactions with the Abl kinase domain. These groups also make the shape of nilotinib very different from that of imatinib. Nilotinib also binds to the kinase through a large number of weak van der Waals interactions.
Resistance
Nilotinib has shown effect against most mutations (32/33) that are associated with imatinib resistance but the T315I mutant remains resistant to nilotinib. Its ineffectiveness against the T315I mutant seems to be a consequence of the loss of an H-bond interaction between threonine-O and aniline-NH on nilotinib and a steric clash between the isoleucine-methyl group and 2-methylphenyl phenyl group of nilotinib. On the other hand, resistance to nilotinib is associated with a limited spectrum of Bcr-Abl kinase mutations that mostly affect the P-loop and T315I. However all mutations except T315I were effectively suppressed by increasing nilotinib concentration. Although nilotinib is more potent than imatinib it is possible that its specific mode of binding to Abl may make other sites vulnerable to new kinds of drug resistance.Dasatinib (BMS-354825)
Development
Dasatinib
Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL). Specifically it is used to treat cases that ar ...
is a thiazolylaminopyrimidine developed as the hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative n ...
salt. It was discovered with a program directed towards immunosuppressive drugs
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent activity of the immune system.
Classification
Immunosuppressive drugs can be classified into ...
and is 325-fold more potent against cells expressing wild type Bcr-Abl than imatinib. Dasatinib is a multi targeted inhibitor of Bcr-Abl and Src family kinases. It also has inhibitory activity against additional downstream kinases.

Binding
Dasatinib binds to Abl with less stringent conformational requirements than imatinib so it exhibits increased potency but reduced selectivity compared to imatinib. Dasatinib binds both the active and inactive conformation of Abl kinase, contrary to the binding of most other TKIs to the active form only. Compounds that target the active conformation have been identified but the binding site in all the hundreds of human protein kinases is very similar. Therefore, there is a considerably greater scope for dissimilarities between the inactive conformations so the efforts to discover highly selective kinase inhibitors are being directed towards molecules that bind to the inactive conformation. Dasatinib has some structural elements in common with nilotinib, in particular the juxtaposition of the aminopyrimidine and thecarboxamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
groups. The aminothiazole segment of dasatinib makes a bi-dentate H-bonding interaction with the backbone CO and NH of Met-318 and the amide-NH makes an H-bond with the side chain oxygen of Thr-315.
Resistance
Since dasatinib is an inhibitor of Src family kinases, it can overcome resistance due to Src family kinase activation. Because it does not bind to Bcr-Abl with the same stringent conformational requirements as imatinib, it can inhibit all Bcr-Abl kinase domain mutants except for T315I. Dasatinib is also not a substrate of multidrug P-glycoprotein efflux pumps like imatinib. Because of this dasatinib may be active in some patients after failure with both imatinib and nilotinib. Although dasatinib is much more potent than imatinib it is possible, like with nilotinib, that its specific mode of binding to Abl may lead to new vulnerable sites that could confer new kinds of drug resistance. Mutations have been found on Phe317 so that is a potential vulnerable site for this drug.Bosutinib (SKI-606)
Development
 Bosutinib's structure is based on a
Bosutinib's structure is based on a quinoline
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only sli ...
scaffold and is structurally related to the AstraZeneca quinazoline
Quinazoline is an organic compound with the formula C8H6N2. It is an aromatic heterocycle with a bicyclic structure consisting of two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring. It is a light yellow crystalline solid ...
template. Src kinase dependent yeast screening led to characterization of a 4-anilino-3-quinoline carbonitrile as an Src inhibitor. Combination of the features of this hit and a related compound, and attachment of solubilizing
Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto micelles. Solublization may occur in a system consisting of a solvent, an association colloid (a c ...
groups, led to the discovery of bosutinib. It was suggested to be an Abl kinase inhibitor and when tested as such it turned out to be slightly more potent against Abl than Src (IC50
The half maximal inhibitory concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function. IC50 is a quantitative measure that indicates how much of a particular inhibitory substance ...
1,4 nM vs. 3,5 nM). Bosutinib's activity was first described in 2001 and it was disclosed as an Abl kinase inhibitor in 2003. At first it was believed that bosutinib was a selective Src kinase inhibitor but now it is known that its kinase inhibition profile is far less restricted than originally thought. Bosutinib inhibits Src, Abl and a wide range of both tyrosine and serine-threonine kinases.
Resistance
Bosutinib inhibited cells expressing a variety of mutations, some of which led to imatinib resistance, but the T315 mutation was completely resistant to bosutinib. In contrast to imatinib, nilotinib and dasatinib, bosutinib is not an efficient substrate for multidrug resistance (MDR) transporters that promotes efflux of foreign molecules from cells. Bosutinib even inhibits these transporter proteins in higher concentrations.Ponatinib (AP24534)
ARIAD Pharmaceuticals
ARIAD Pharmaceuticals, Inc. was an American oncology company, now part of Takeda Oncology, which was founded in 1991 by Harvey J. Berger, M.D. and headquartered in Cambridge, Massachusetts. ARIAD engaged in the discovery, development, and commer ...
, Inc. announced on September 10, 2010 that ponatinib
Ponatinib (trade name Iclusig , previously AP24534) is an oral drug developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a ...
, an orally active Bcr-Abl TKI effective against the T315I mutation had been approved for a phase II clinical trial.
The road to discovery can be linked to AP23464, one of the first of Ariad's ATP competitive dual Src/Abl inhibitors. AP23464 was identified using structure base drug design and focused synthetic libraries of trisubstituted purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines ...
analogs. The substance potently inhibits, on nanomolar scale, Src and Bcr-Abl kinases including many common imatinib resistant Bcr-Abl mutations. AP23464 does not inhibit the T315I mutation, however, whereas AP24534 (ponatinib) does.
Development
aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
amide structure, that was known to have a high affinity to the inactive conformation by forming crucial hydrogen bonds and filling hydrophobic pockets on the kinase. Furthermore, it was determined that the cyclopentyl group on the purine core clashed with a glycine rich P-loop in that confirmation and was thus removed from the molecule. Then with in-vitro testing on inhibitory activity and in-vivo oral absorption assays a more lipophilic, amide bound, cyclopropyl
A cyclopropyl group is a chemical structure derived from cyclopropane, and can participate in organic reactions that constitute cycloadditions and rearrangement organic reactions of cyclopropane. The group has an empirical formula of C3H5 and che ...
group on C6 on the purine core was found to display both satisfactory pharmacokinetics and efficacy. Finally modifications on the diarylamide side chain by adding imidazole appendages were inspired by then newly released nilotinib structure. Those modifications resulted in what was called AP24163. During this development cycle, Ariad tested several substances against cells transfected with T315I mutated Bcr-Abl kinase and, surprisingly, found AP24163 demonstrated reasonable inhibitory action on top of potent inhibition of native Bcr-Abl.
Following up on that breakthrough Ariad began further research to increase the efficacy of compound AP24163 against the T315I mutation. Docking of the molecule into the ATP binding site of T315I mutated Bcr-Abl kinase revealed that the expected steric clash with isoleucine was not present due to a lesser sterically demanding vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
linkage between the purine core and the diarylamide side chain compared to other TKIs. The first step was to try to find an even less sterically demanding structure. First an acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
linkage was tested, that resulted in higher potency but unfavorable pharmacokinetics. Later, a more stable 2-butyne
2-Butyne (dimethylacetylene, crotonylene or but-2-yne) is an alkyne with chemical formula CH3C≡CCH3. Produced artificially, it is a colorless, volatile, pungent liquid at standard temperature and pressure.
2-Butyne is of interest to physical c ...
linkage was selected. To achieve this linkage an imidazol,2-a
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline o ...
yridine core was used as a starting material for a Sonogashira reaction; but the pharmacokinetics were still poor. When developing AP24163, adding a cyclopropane side chain on C8 in the purine core resulted in favorable pharmacokinetics. Several different side chains were then tested, but the best results were obtained with no side chain at all, resulting a substance with satisfactory pharmacokinetics, but now with reduced potency against T315I also. The first step in increasing the potency again was to look at other TKI's. Imatinib has a terminal methyl piperazine group which has been shown to form a hydrogen bond with the carbonyl oxygen atom of residue Ile-360 in the activation loop of the Abl kinase. The piperazine ring is also a common solubilizing group that could further improve the pharmacokinetic properties of the molecule. Those speculations were confirmed with a two-fold increase in inhibitory action against Bcr-Abl T315I mutated kinase and the silver lining was the plasma protein binding of the substance (named '19a') appeared to have decreased, allowing for smaller doses with the same potency. Whilst '19a' exhibited good oral pharmacokinetics in both mice and rats, it also retained high partition coefficient
In the physical sciences, a partition coefficient (''P'') or distribution coefficient (''D'') is the ratio of concentrations of a compound in a mixture of two immiscible solvents at equilibrium. This ratio is therefore a comparison of the solub ...
at 6.69. So, in attempts to reduce the molecule's lipophilicity further, substitution of a single carbon atom on the imidazo,2-a
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline o ...
yridine core was made; which resulted in what is now known as the compound ponatinib.
Binding
X-ray crystallographic analysis of ponatinib and T315I Bcr-Abl mutated kinase display that the imidazo ,2b yridazine core rests in the adenine pocket of the enzyme. The methylphenyl group occupies a hydrophobic pocket behind I315, theethynyl In organic chemistry, the term ethynyl designates a functional group with a double bond with 2 carbon atoms both with
sp hybridisation and a triple bond(1 sigma and 2 pi bond) . It is a species similar to acetylene (or in IUPAC ethyne ) with a less ...
linkage forms favorable van der Waals interactions with the amino acid and the trifluoromethyl group binds to a pocket induced by the inactive conformation kinase. Also in the conformation of the kinase that ponatinb rests in, additional favorable van der Waals interactions between the drug and Tyr-253 and Phe-382. Five hydrogen bonds are generated, with the backbone of Met-318 in the hinge region, with the backbone of Asp-381, with the side chain of Glu-286 and the protonated methylpiperazine with the backbone-carbonyl atoms of Ile-360 and His-361.
With this structure ponatinib has been shown to have a relatively broad kinase specificity profile which can probably be linked to the linearity of the linkage section of the molecule. With this linear structure the drug appears to avoid steric clashes with hydrophobic TK gatekeeper residues. Despite, or even because of this, ponatinib is a potent drug and targets not just most of the known mutations on the Bcr-Abl TK but, most importantly of all, T315I. This mutation is emerging as a common pathway to failure of both first and second line treatments. Unlike other T315I targeting inhibitors in development, ponatinib does not target Aurora kinases, which clearly distinguishes it from them and emphasizes the significance of its discovery.
Bafetinib (INNO-406)
With the emerging resistance to imatinib treatment after its launch alternative treatment was highly sought after.Bafetinib
Bafetinib (NS-187) is an experimental cancer drug developed by Nippon Shinyaku and licensed to CytRx. It is an inhibitor of Lyn and Bcr-Abl. It reached phase II clinical trials in 2010.
Development
Imatinib was the first Bcr-Abl tyrosine-k ...
was the offspring of an attempt to create a more potent drug than imatinib, with efficacy against various point mutations in the Bcr-Abl kinase, with fewer adverse effects and with narrower kinase spectra, namely just Lyn and Bcr-Abl.
Development
In the search for a substance that fit the criteria mentioned, the crystal structure of imatinib bound to Abl was examined. This revealed a hydrophobic pocket around the phenyl ring adjacent to the piperazinylmethyl group of imatinib. Attempts to utilize this pocket to increase efficacy led to the addition of various hydrophobic groups including singlefluoro
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, ...
, bromo and chloro
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
substituents. Finally a trifluoromethyl group at position 3 was found to give the best results, with approximately 36-fold improvement over imatinib. The addition of a hydrophobic group now needed to be countered to sustain the solubility of the substance. Closer examination of the crystal structure of imatinib-kinase complex revealed Tyr-236 was in close proximity to the pyridine ring of imatinib, suggesting there was little or no room for a larger group there. With that in mind a more hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
pyrimidine ring was substituted for the pyridine, which was found to increase solubility while leaving efficacy the same or even slightly greater. Finally to improve the hydrogen bonding of the piperazine ring of imatinib with Ile-360 and His-361, pyrrolidine and azetidine derivatives were introduced. The most promising substance from these final modifications was labeled NS-187.
Binding
1,3,4 thiadiazole derivatives - Substance 14
Some interest has been with thiazol and thiadiazole derivatives and their ability to inhibit Bcr-Abl TKs.Development
thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
ring. Though it has to be noted this analysis was done with comparing the crystal structure of Abl and dasatinib, which is the inactive conformation of Abl, the knowledge gathered from the docking and structure analysis led to identification of a compound, referred to as substance 14, with a high affinity to Abl.
Binding
The binding of substance 14 is partly similar to dasatinib, the aminothiazole segment of substance 14 makes a bi-dentate H-bonding interaction with the backbone CO and NH of Met-318 while themethoxy
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula .
On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position a ...
-benzene falls nicely into a hydrophobic pocket created by Val 256, Ala 253, Lys 271 and Ala 380. Whilst the similar binding properties to those of dasatinib, suggests the possibility of producing Bcr-Abl TKI's from thiazole cores is real, the question remains open whether this research will just lead to a dasatinib analog or a novel way to inhibit TKs.
Others
Rebastinib (DCC-2036) Also an inhibitor of TIE-2 and VEGFR-2. It has had a phase 1 clinical trial for Leukemias (Ph+ CML With T315I Mutation). It is in a phase 1 clinical trial of combination therapy for metastatic breast cancer. Asciminib (ABL001) is an inhibitor of the Abelson kinase targeting the myristoyl pocket to allosterically inhibit the enzyme. As of August 2020, it had completed a phase III study in CML (ASCEMBL) showing superior efficacy to bosutinib.Summary
Current status - re Ph+ CML
Imatinib remains a standard frontline TKI. Nilotinib and dasatinib are also approved by the FDA as frontline drugs, in June and October 2010, respectively. Four of these drugs, nilotinib, dasatinib, bosutinib and ponatinib are approved for the treatment of imatinib-resistant or intolerant CML. The first-line data for these compounds are encouraging and suggest that some or all of them may replace imatinib as a frontline standard TKI in the future.References
{{reflist, colwidth=30em, refs= Druker, B. J. and Lydon, N. B. (2000). "Lessons learned from the development of an Abl tyrosine kinase inhibitor for chronic myelogenous leukemia". ''The journal of Clinical Investigation'': 3-7. Stein, B., Smith, B.D. (2010). "Treatment Options for Patients With Chronic Myeloid Leukemia Who Are Resistant to or Unable to Tolerate Imatinib". ''Clinical Therapeutics'': 804-820. Manley, P.W., Cowan-Jacob, S. W., Buchdunger, E., Fabbro, D., Fendrich, G., Furet, P., Meyer, T. and Zimmermann, J. (2002). "Imatinib: a selective tyrosine kinase inhibitor". ''European Journal of Cancer'': S19-S27. Buchanan, S. G. (2003) "Protein structure: discovering selective protein kinase inhibitors". ''Targets'': 101-108. Shawver, L. K., Slamon, D. and Ullrich, A. (2002). "Smart drugs:Tyrosine kinase inhibitors in cancer therapy". ''Cancer Cell'': 117-123. Bixby, D., Talpaz, M. (2009). "Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance". ''Hematology'': 461-476. {{cite journal , last1 = Gorre , first1 = M. , last2 = Mohammed , first2 = M. , last3 = Ellwood , first3 = K. , last4 = Hsu , first4 = N. , last5 = Paquette , first5 = R. , last6 = Rao , first6 = P. N. , last7 = Sawyers , first7 = C. L. , s2cid = 1279564 , year = 2001 , title = Clinical Resistance to STI-571 Cancer Therapy Caused by BCR-ABL Gene Mutation or Amplification , journal = Science , volume = 293, issue = 5531, pages = 876–880 , doi = 10.1126/science.1062538 , pmid = 11423618 {{cite journal , last1 = Jabbour , first1 = E. , last2 = Cortes , first2 = J. , last3 = Kantarjian , first3 = H. , year = 2009 , title = Nilotinib for the treatment of chronic myeloid leukemia: An evidence-based review , journal = Core Evidence , volume = 4 , pages = 207–213 , pmid = 20694077 , pmc = 2899790 , doi = 10.2147/CE.S6003 {{Cite journal , last1 = Valent , first1 = P. , title = Standard treatment of Ph+ CML in 2010: how, when and where not to use what BCR/ABL1 kinase inhibitor? , journal = European Journal of Clinical Investigation , volume = 40 , issue = 10 , pages = 918–931 , year = 2010 , pmid = 20597967 , doi = 10.1111/j.1365-2362.2010.02328.x, doi-access = free {{Cite journal, last1 = Olivieri , first1 = A., last2 = Manzione , first2 = L., title = Dasatinib: a new step in molecular target therapy, journal = Annals of Oncology, volume = 18, pages = vi42–vi46, year = 2007, pmid = 17591830, doi = 10.1093/annonc/mdm223, doi-access = free {{Cite journal, last1 = Manley , first1 = P., last2 = Stiefl , first2 = N., last3 = Cowan-Jacob , first3 = S., last4 = Kaufman , first4 = S., last5 = Mestan , first5 = J., last6 = Wartmann , first6 = M., last7 = Wiesmann , first7 = M., last8 = Woodman , first8 = R., last9 = Gallagher , first9 = N., title = Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib, journal = Bioorganic & Medicinal Chemistry, volume = 18, issue = 19, pages = 6977–6986, year = 2010, pmid = 20817538, doi = 10.1016/j.bmc.2010.08.026 {{Cite journal, last1 = Radi , first1 = M., last2 = Crespan , first2 = E., last3 = Botta , first3 = G., last4 = Falchi , first4 = F., last5 = Maga , first5 = G., last6 = Manetti , first6 = F., last7 = Corradi , first7 = V., last8 = Mancini , first8 = M., last9 = Santucci , first9 = M., last10 = Schenone , first10 = S., last11 = Botta , first11 = M., title = Discovery and SAR of 1,3,4-thiadiazole derivatives as potent Abl tyrosine kinase inhibitors and cytodifferentiating agents, journal = Bioorganic & Medicinal Chemistry Letters, volume = 18, issue = 3, pages = 1207–1211, year = 2008, pmid = 18078752, doi = 10.1016/j.bmcl.2007.11.112, hdl = 11381/2432276, hdl-access = free {{Cite journal, last1 = Manetti , first1 = F., last2 = Falchi , first2 = F., last3 = Crespan , first3 = E., last4 = Schenone , first4 = S., last5 = Maga , first5 = G., last6 = Botta , first6 = M., title = N-(thiazol-2-yl)-2-thiophene carboxamide derivatives as Abl inhibitors identified by a pharmacophore-based database screening of commercially available compounds, journal = Bioorganic & Medicinal Chemistry Letters, volume = 18, issue = 15, pages = 4328–4331, year = 2008, pmid = 18621522, doi = 10.1016/j.bmcl.2008.06.082 {{Cite journal, last1 = Kimura , first1 = S., last2 = Naito , first2 = H., last3 = Segawa , first3 = H., last4 = Kuroda , first4 = J., last5 = Yuasa , first5 = T., last6 = Sato , first6 = K., last7 = Yokota , first7 = A., last8 = Kamitsuji , first8 = Y., last9 = Kawata , first9 = E., last10 = Ashihara , first10 = E., last11 = Nakaya , first11 = Y., last12 = Naruoka , first12 = H., last13 = Wakayama , first13 = T., last14 = Nasu , first14 = K., last15 = Asaki , first15 = T., last16 = Niwa , first16 = T., last17 = Hirabayashi , first17 = K., last18 = Maekawa , first18 = T., title = NS-187, a potent and selective dual Bcr-Abl/Lyn tyrosine kinase inhibitor, is a novel agent for imatinib-resistant leukemia, journal = Blood, volume = 106, issue = 12, pages = 3948–3954, year = 2005, pmid = 16105974, doi = 10.1182/blood-2005-06-2209 {{Cite journal, last1 = Asaki , first1 = T., last2 = Sugiyama , first2 = Y., last3 = Hamamoto , first3 = T., last4 = Higashioka , first4 = M., last5 = Umehara , first5 = M., last6 = Naito , first6 = H., last7 = Niwa , first7 = T., title = Design and synthesis of 3-substituted benzamide derivatives as Bcr-Abl kinase inhibitors, journal = Bioorganic & Medicinal Chemistry Letters, volume = 16, issue = 5, pages = 1421–1425, year = 2006, pmid = 16332440, doi = 10.1016/j.bmcl.2005.11.042 {{Cite journal, last1 = Horio , first1 = T., last2 = Hamasaki , first2 = T., last3 = Inoue , first3 = T., last4 = Wakayama , first4 = T., last5 = Itou , first5 = S., last6 = Naito , first6 = H., last7 = Asaki , first7 = T., last8 = Hayase , first8 = H., last9 = Niwa , first9 = T., title = Structural factors contributing to the Abl/Lyn dual inhibitory activity of 3-substituted benzamide derivatives, journal = Bioorganic & Medicinal Chemistry Letters, volume = 17, issue = 10, pages = 2712–2717, year = 2007, pmid = 17376680, doi = 10.1016/j.bmcl.2007.03.002 {{Cite journal, last1 = Deguchi , first1 = Y., last2 = Kimura , first2 = S., last3 = Ashihara , first3 = E., last4 = Niwa , first4 = T., last5 = Hodohara , first5 = K., last6 = Fujiyama , first6 = Y., last7 = Maekawa , first7 = T., title = Comparison of imatinib, dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines, journal = Leukemia Research, volume = 32, issue = 6, pages = 980–983, year = 2008, pmid = 18191450, doi = 10.1016/j.leukres.2007.11.008 {{Cite journal, last1 = An , first1 = X., last2 = Tiwari , first2 = A., last3 = Sun , first3 = Y., last4 = Ding , first4 = P., last5 = Ashby Jr , first5 = C., last6 = Chen , first6 = Z., title = BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review, journal = Leukemia Research, volume = 34, issue = 10, pages = 1255–1268, year = 2010, pmid = 20537386, doi = 10.1016/j.leukres.2010.04.016 {{Cite journal, last1 = O'Hare , first1 = T., last2 = Shakespeare , first2 = W., last3 = Zhu , first3 = X., last4 = Eide , first4 = C., last5 = Rivera , first5 = V., last6 = Wang , first6 = F., last7 = Adrian , first7 = L., last8 = Zhou , first8 = T., last9 = Huang , first9 = W., last10 = Xu , first10 = Q., last11 = Metcalf Ca , first11 = C. A., last12 = Tyner , first12 = J. W., last13 = Loriaux , first13 = M. M., last14 = Corbin , first14 = A. S., last15 = Wardwell , first15 = S., last16 = Ning , first16 = Y., last17 = Keats , first17 = J. A., last18 = Wang , first18 = Y., last19 = Sundaramoorthi , first19 = R., last20 = Thomas , first20 = M., last21 = Zhou , first21 = D., last22 = Snodgrass , first22 = J., last23 = Commodore , first23 = L., last24 = Sawyer , first24 = T. K., last25 = Dalgarno , first25 = D. C., last26 = Deininger , first26 = M. W. N., last27 = Druker , first27 = B. J., last28 = Clackson , first28 = T., title = AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance, journal = Cancer Cell, volume = 16, issue = 5, pages = 401–412, year = 2009, pmid = 19878872, pmc = 2804470, doi = 10.1016/j.ccr.2009.09.028 {{Cite journal , last1 = Huang , first1 = W. S. , last2 = Metcalf , first2 = C. A. , last3 = Sundaramoorthi , first3 = R. , last4 = Wang , first4 = Y. , last5 = Zou , first5 = D. , last6 = Thomas , first6 = R. M. , last7 = Zhu , first7 = X. , last8 = Cai , first8 = L. , last9 = Wen , first9 = D., title = Discovery of 3- -(Imidazo[1,2-byridazin-3-yl)ethynyl.html" ;"title=",2-b.html" ;"title="-(Imidazo[1,2-b">-(Imidazo[1,2-byridazin-3-yl)ethynyl">,2-b.html" ;"title="-(Imidazo[1,2-b">-(Imidazo[1,2-byridazin-3-yl)ethynyl4-methyl-N-