Barton–McCombie deoxygenation on:
[Wikipedia]
[Google]
[Amazon]
The Barton–McCombie deoxygenation is an  This deoxygenation reaction is a radical substitution. In the related
This deoxygenation reaction is a radical substitution. In the related
 In this
In this 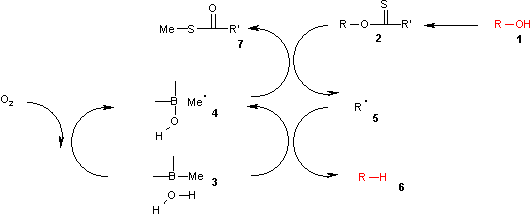 It is found by theoretical calculations that an O-H homolysis reaction in the borane-water complex is
It is found by theoretical calculations that an O-H homolysis reaction in the borane-water complex is
Barton-McCombie @ organic-chemistry.org
*
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
in which a hydroxy functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
in an organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
is replaced by a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
to give an alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
. It is named after British chemists Sir Derek Harold Richard Barton and Stuart W. McCombie.
Barton decarboxylation
The Barton decarboxylation is a radical reaction in which a carboxylic acid is converted to a thiohydroxamate ester (commonly referred to as a Barton ester). The product is then heated in the presence of a radical initiator and a suitable hydroge ...
the reactant is a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
.
Mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
consists of a catalytic radical initiation step and a propagation step. The alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
(1) is first converted into a reactive carbonothioyl intermediate such as a thionoester or xanthate
150px, Sodium salt of ethyl xanthate
Xanthate usually refers to a salt with the formula (R = alkyl; M+ = Na+, K+), thus they are the metal-thioate/''O''-esters of dithiocarbonate. The name ''xanthates'' is derived from Ancient Greek ''xanthos' ...
2. Heating of AIBN results in its homolytic cleavage, generating two 2-cyanoprop-2-yl radicals 9 which each abstract a proton from tributylstannane 3 to generate tributylstannyl radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
4 and inactive 10. The tributyltin radical abstracts the xanthate group from 2 by attack of 4 at the sulfur atom with concurrent homolytic cleavage of the C-S π bond. This leaves a carbon centered radical that forms a C-O π bond through homolytic cleavage of the R-O σ bond, giving alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
radical 5 and tributyltin xanthate 7. The sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
tin bond in this compound is very stable and provides the driving force for this reaction. The alkyl radical 5 then abstracts a hydrogen atom from a new molecule of tributylstannane generating the desired deoxygenated product (6) and a new radical species ready for propagation.
Variations
Alternative hydrogen sources
The main disadvantage of this reaction is the use of tributylstannane which is toxic, expensive and difficult to remove from the reaction mixture. One alternative is the use oftributyltin oxide
Tributyltin oxide (TBTO) is an organotin compound chiefly used as a biocide (fungicide and molluscicide), especially a wood preservative. Its chemical formula is C4H9)3Snsub>2O. It is a colorless viscous liquid. It is poorly soluble in water (20 ...
as the radical source and poly(methylhydridesiloxane) (PMHS) as the hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
source. Phenyl chlorothionoformate used as the starting material ultimately generates carbonyl sulfide
Carbonyl sulfide is the chemical compound with the linear formula OCS. It is a colorless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom. Carbonyl sulfide can be consi ...
.
Trialkyl boranes
An even more convenient hydrogen donor is provided by trialkylborane-water complexes such astrimethylborane
Trimethylborane (TMB) is a toxic, Pyrophoricity, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).
Properties
As a liquid it is colourless. The strongest line in the infrared spectrum is at ...
contaminated with small amounts of water.
catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
the reaction is initiated by air oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
of the trialkylborane 3 by air to the methyl radical 4. This radical reacts with the xanthate 2 to S-methyl-S-methyl dithiocarbonate 7 and the radical intermediate 5. The (CH3)3B.H2O complex 3 provides a hydrogen for recombining with this radical to the alkane 6 leaving behind diethyl borinic acid and a new methyl radical.
 It is found by theoretical calculations that an O-H homolysis reaction in the borane-water complex is
It is found by theoretical calculations that an O-H homolysis reaction in the borane-water complex is endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
with an energy similar to that of the homolysis reaction in tributylstannane but much lower than the homolysis reaction of pure water.
Scope
A variation of this reaction was used as one of the steps in thetotal synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes i ...
of azadirachtin
Azadirachtin, a chemical compound belonging to the limonoid group, is a secondary metabolite present in neem seeds. It is a highly oxidized tetranortriterpenoid which boasts a plethora of oxygen-bearing functional groups, including an enol ether, ...
:
:
In another variation the reagent is the imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...
1,1'-thiocarbonyldiimidazole (TCDI), for example in the total synthesis of pallescensin B. TCDI is especially good to primary alcohols because there is no resonance stabilization of the xanthate because the nitrogen lonepair is involved in the aromatic sextet.
:
The reaction also applies to ''S''-alkylxanthates. With triethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane.
Pr ...
as a novel metal-free reagent, the required hydrogen atoms are abstracted from protic solvents, the reactor wall or even (in strictly anhydrous conditions) the borane itself.''Part 2. Mechanistic aspects of the reduction of ''S''-alkyl-thionocarbonates in the presence of triethylborane and air'' Allais F, Boivin J, Nguyen V Beilstein J. Org. Chem., 2007 3:45 ( 12 December 2007 )
See also
* Chugaev eliminationReferences
External links
Barton-McCombie @ organic-chemistry.org
*
Chemical & Engineering News
''Chemical & Engineering News'' (''C&EN'') is a weekly news magazine published by the American Chemical Society, providing professional and technical news and analysis in the fields of chemistry and chemical engineering. article on alkylborane reaction
{{DEFAULTSORT:Barton-McCombie deoxygenation Free radical reactions Organic redox reactions Name reactions
{{DEFAULTSORT:Barton-McCombie deoxygenation Free radical reactions Organic redox reactions Name reactions