Arsenic DNA on:
[Wikipedia]
[Google]
[Amazon]
 Arsenic biochemistry refers to biochemical processes that can use
Arsenic biochemistry refers to biochemical processes that can use
 The evidence that arsenic may be a beneficial nutrient at trace levels below the background to which living organisms are normally exposed has been reviewed. Some organoarsenic compounds found in nature are arsenobetaine and arsenocholine, both being found in many marine organisms. Some As-containing
The evidence that arsenic may be a beneficial nutrient at trace levels below the background to which living organisms are normally exposed has been reviewed. Some organoarsenic compounds found in nature are arsenobetaine and arsenocholine, both being found in many marine organisms. Some As-containing
File: ArsenobetainePIC.svg, Arsenobetaine, one of the most common arsenic compound in nature. Also common is arsenocholine, which has CH2OH in place of CO2H).
File: Trimethylarsine-2D.png,
A topical source of arsenic are the green pigments once popular in wallpapers, e.g. Paris green. A variety of illness have been blamed on this compound, although its toxicity has been exaggerated.
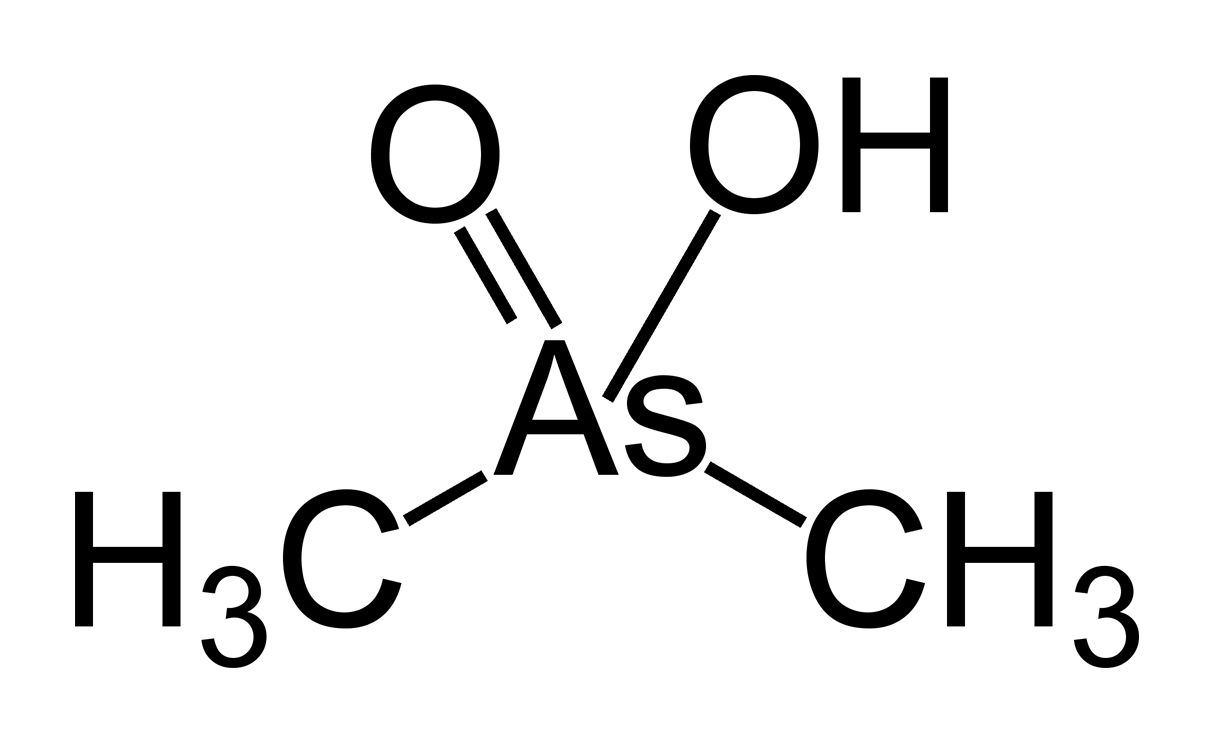 In mammals, methylation occurs in the liver by methyltransferases, the products being the (CH3)2AsOH ( dimethylarsinous acid) and (CH3)2As(O)OH ( dimethylarsinic acid), which have the oxidation states As(III) and As(V), respectively. Although the mechanism of methylation of arsenic in humans has not been elucidated, the source of methyl is methionine, which suggests a role of
In mammals, methylation occurs in the liver by methyltransferases, the products being the (CH3)2AsOH ( dimethylarsinous acid) and (CH3)2As(O)OH ( dimethylarsinic acid), which have the oxidation states As(III) and As(V), respectively. Although the mechanism of methylation of arsenic in humans has not been elucidated, the source of methyl is methionine, which suggests a role of
 Arsenic biochemistry refers to biochemical processes that can use
Arsenic biochemistry refers to biochemical processes that can use arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, ...
or its compounds, such as arsenate
The arsenate ion is .
An arsenate (compound) is any compound that contains this ion. Arsenates are salts or esters of arsenic acid.
The arsenic atom in arsenate has a valency of 5 and is also known as pentavalent arsenic or As(V).
Arsenate res ...
. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compound
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fu ...
s are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and ...
s. This pattern is general for other related elements, including selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifer
An aquifer is an underground layer of water-bearing, permeable rock, rock fractures, or unconsolidated materials ( gravel, sand, or silt). Groundwater from aquifers can be extracted using a water well. Aquifers vary greatly in their characteris ...
s, potentially affecting many millions of people via biochemical processes.Elke Dopp, Andrew D. Kligerman and Roland A. Diaz-Bone Organoarsenicals. Uptake, Metabolism, and Toxicity 2010, Royal Society of Chemistry. .
Sources of arsenic
Organoarsenic compounds in nature
 The evidence that arsenic may be a beneficial nutrient at trace levels below the background to which living organisms are normally exposed has been reviewed. Some organoarsenic compounds found in nature are arsenobetaine and arsenocholine, both being found in many marine organisms. Some As-containing
The evidence that arsenic may be a beneficial nutrient at trace levels below the background to which living organisms are normally exposed has been reviewed. Some organoarsenic compounds found in nature are arsenobetaine and arsenocholine, both being found in many marine organisms. Some As-containing nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleoti ...
s (sugar derivatives) are also known. Several of these organoarsenic compounds arise via methylation processes. For example, the mold '' Scopulariopsis brevicaulis'' produces significant amounts of trimethylarsine
Trimethylarsine (abbreviated TMA or TMAs) is the chemical compound with the formula (CH3)3As, commonly abbreviated As Me3 or TMAs. This organic derivative of arsine has been used as a source of arsenic in microelectronics industry, a building bloc ...
if inorganic arsenic is present. The organic compound arsenobetaine is found in some marine foods such as fish and algae, and also in mushrooms in larger concentrations. In clean environments, the edible mushroom species '' Cyanoboletus pulverulentus'' hyperaccumulates arsenic in concentrations reaching even 1,300 mg/kg in dry weight; cacodylic acid
Cacodylic acid is an organoarsenic compound with the formula (CH3)2 AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water.
Neutralization of cacodylic acid with base gives cac ...
is the major As compound. A very unusual composition of organoarsenic compounds was found in deer truffles ('' Elaphomyces'' spp.). The average person's intake is about 10–50 µg/day. Values about 1000 µg are not unusual following consumption of fish or mushrooms; however, there is little danger in eating fish since this arsenic compound is nearly non-toxic.
Trimethylarsine
Trimethylarsine (abbreviated TMA or TMAs) is the chemical compound with the formula (CH3)3As, commonly abbreviated As Me3 or TMAs. This organic derivative of arsine has been used as a source of arsenic in microelectronics industry, a building bloc ...
, produced by microbial action on arsenate
The arsenate ion is .
An arsenate (compound) is any compound that contains this ion. Arsenates are salts or esters of arsenic acid.
The arsenic atom in arsenate has a valency of 5 and is also known as pentavalent arsenic or As(V).
Arsenate res ...
-derived pigments
File: DM-Oxoarsenosugars.png, Arsenic-containing ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compo ...
derivatives (R = several groups)
Trimethylarsine
Trimethylarsine (abbreviated TMA or TMAs) is the chemical compound with the formula (CH3)3As, commonly abbreviated As Me3 or TMAs. This organic derivative of arsine has been used as a source of arsenic in microelectronics industry, a building bloc ...
, once known as Gosio's gas, is an intensely malodorous organoarsenic compound that is commonly produced by microbial action on inorganic arsenic substrates.
Arsenic (V) compounds are easily reduced to arsenic (III) and could have served as an electron acceptor on primordial Earth. Lakes that contain a substantial amount of dissolved inorganic arsenic, harbor arsenic-tolerant biota.
Incorrect claims of arsenic-based life (phosphorus substitution)
Although phosphate and arsenate are structurally similar, there is no evidence that arsenic replaces phosphorus in DNA or RNA. A 2010 experiment involving the bacteria GFAJ-1 that made this claim was refuted by 2012.Anthropogenic arsenic compounds
Anthropogenic (man-made) sources of arsenic, like the natural sources, are mainly arsenic oxides and the associated anions. Man-made sources of arsenic, include wastes from mineral processing, swine and poultry farms. For example, many ores, especiallysulfide mineral
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, th ...
s, are contaminated with arsenic, which is released in roasting (burning in air). In such processing, arsenide
In chemistry, an arsenide is a compound of arsenic with a less electronegative element or elements. Many metals form binary compounds containing arsenic, and these are called arsenides. They exist with many stoichiometries, and in this respect a ...
is converted to arsenic trioxide
Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, whose major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication, it ...
, which is volatile at high temperatures and is released into the atmosphere. Poultry and swine farms make heavy use of the organoarsenic compound roxarsone as an antibiotic in feed. Some wood is treated with copper arsenates as a preservative. The mechanisms by which these sources affect "downstream" living organisms remains uncertain but are probably diverse. One commonly cited pathway involves methylation.
The monomethylated acid, methanearsonic acid (CH3AsO(OH)2), is a precursor to fungicides (tradename Neoasozin) in the cultivation of rice and cotton. Derivatives of phenylarsonic acid
Phenylarsonic acid is the chemical compound with the formula C6H5AsO(OH)2, commonly abbreviated PhAsO3H2. This colourless solid is an organic derivative of arsenic acid, AsO(OH)3, where one OH group has been replaced by a phenyl group. The compo ...
(C6H5AsO(OH)2) are used as feed additives for livestock, including 4-hydroxy-3-nitrobenzenearsonic acid
Roxarsone is an organoarsenic compound that has been used in poultry production as a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat. As of June 2011, it was approved for chicken feed in the United States ...
(3-NHPAA or Roxarsone), ureidophenylarsonic acid, and ''p''-arsanilic acid. These applications are controversial as they introduce soluble forms of arsenic into the environment.
Arsenic-based drugs
Despite, or possibly because of, its long-known toxicity, arsenic-containing potions and drugs have a history inmedicine
Medicine is the science and practice of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care pr ...
and quackery
Quackery, often synonymous with health fraud, is the promotion of fraudulent or ignorant medical practices. A quack is a "fraudulent or ignorant pretender to medical skill" or "a person who pretends, professionally or publicly, to have skill, ...
that continues into the 21st century. Starting in the early 19th century and continuing into the 20th century, Fowler's solution, a toxic concoction of sodium arsenite, was sold. The organoarsenic compound Salvarsan
Arsphenamine, also known as Salvarsan or compound 606, is a drug that was introduced at the beginning of the 1910s as the first effective treatment for syphilis, relapsing fever, and African trypanosomiasis.
This organoarsenic compound was the fi ...
was the first synthetic chemotherapeutic agent
This is a list of chemotherapeutic agents, also known as cytotoxic agents or cytostatic drugs, that are known to be of use in chemotherapy for cancer. This list is organized by type of agent, although the subsections are not necessarily definitive ...
, discovered by Paul Ehrlich
Paul Ehrlich (; 14 March 1854 – 20 August 1915) was a Nobel Prize-winning German physician and scientist who worked in the fields of hematology, immunology, and antimicrobial chemotherapy. Among his foremost achievements were finding a cure ...
. The treatment, however, led to many problems causing long lasting health complications.Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. Around 1943 it was finally superseded by penicillin.
The related drug Melarsoprol
Melarsoprol is an arsenic-containing medication used for the treatment of sleeping sickness (African trypanosomiasis). It is specifically used for second-stage disease caused by ''Trypanosoma brucei rhodesiense'' when the central nervous system i ...
is still in use against late-state African trypanosomiasis (sleeping sickness), despite its high toxicity and possibly fatal side effects.
Arsenic trioxide
Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, whose major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication, it ...
(As2O3) inhibits cell growth and induces apoptosis (programmed cell death) in certain types of cancer cells, which are normally immortal and can multiply without limit. In combination with all-trans retinoic acid
Tretinoin, also known as all-''trans'' retinoic acid (ATRA), is a medication used for the treatment of acne and acute promyelocytic leukemia. For acne, it is applied to the skin as a cream, gel or ointment. For leukemia, it is taken by mouth f ...
, it is FDA-approved as first-line treatment for promyelocytic leukemia.
Methylation of arsenic
Inorganic arsenic and its compounds, upon entering thefood chain
A food chain is a linear network of links in a food web starting from producer organisms (such as grass or algae which produce their own food via photosynthesis) and ending at an apex predator species (like grizzly bears or killer whales), de ...
, are progressively metabolised (detoxified) through a process of methylation. The methylation occurs through alternating reductive and oxidative methylation reactions, that is, reduction of pentavalent to trivalent arsenic followed by addition of a methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
(CH3).
 In mammals, methylation occurs in the liver by methyltransferases, the products being the (CH3)2AsOH ( dimethylarsinous acid) and (CH3)2As(O)OH ( dimethylarsinic acid), which have the oxidation states As(III) and As(V), respectively. Although the mechanism of methylation of arsenic in humans has not been elucidated, the source of methyl is methionine, which suggests a role of
In mammals, methylation occurs in the liver by methyltransferases, the products being the (CH3)2AsOH ( dimethylarsinous acid) and (CH3)2As(O)OH ( dimethylarsinic acid), which have the oxidation states As(III) and As(V), respectively. Although the mechanism of methylation of arsenic in humans has not been elucidated, the source of methyl is methionine, which suggests a role of S-adenosyl methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throug ...
. Exposure to toxic doses begin when the liver's methylation capacity is exceeded or inhibited.
There are two major forms of arsenic that can enter the body, arsenic (III) and arsenic (V). Arsenic (III) enters the cells though aquaporin
Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a ...
s 7 and 9, which is a type of aquaglyceroporin. Arsenic (V) compounds use phosphate transporters to enter cells. The arsenic (V) can be converted to arsenic (III) by the enzyme purine nucleoside phosphorylase
Purine nucleoside phosphorylase, PNP, PNPase or inosine phosphorylase () is an enzyme that in humans is encoded by the ''NP'' gene. It catalyzes the chemical reaction
:purine nucleoside + phosphate \rightleftharpoons purine + alpha-D-ribose 1- ...
. This is classified as a bioactivation step, as although arsenic (III) is more toxic, it is more readily methylated.
There are two routes by which inorganic arsenic compounds are methylated. The first route uses Cyt19 arsenic methyltransferase to methylate arsenic (III) to a mono-methylated arsenic (V) compound. This compound is then converted to a mono-methylated arsenic (III) compound using Glutathione S-Transferase Omega-1 ( GSTO1). The mono-methylated arsenic (V) compound can then be methylated again by Cyt19 arsenic methyltransferase, which forms a dimethyl arsenic (V) compound, which can be converted to a dimethyl arsenic (III) compound by Glutathione S-Transferase Omega-1 (GTSO1). The other route uses glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
(GSH) to conjugate with arsenic (III) to form an arsenic (GS) 3 complex. This complex can form a monomethylated arsenic (III) GS complex, using Cyt19 arsenic methyltransferase, and this monomethylated GS complex is in equilibrium with the monomethylated arsenic (III). Cyt19 arsenic methyltransferase can methylate the complex one more time, and this forms a dimethylated arsenic GS complex, which is in equilibrium with a dimethyl arsenic (III) complex. Both of the mono-methylated and di-methylated arsenic compounds can readily be excreted in urine. However, the monomethylated compound was shown to be more reactive and more toxic than the inorganic arsenic compounds to human hepatocyte
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, ...
s (liver), keratinocytes in the skin, and bronchial epithelial cells (lungs).
Studies in experimental animals and humans show that both inorganic arsenic and methylated metabolites cross the placenta
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate mate ...
to the fetus
A fetus or foetus (; plural fetuses, feti, foetuses, or foeti) is the unborn offspring that develops from an animal embryo. Following embryonic development the fetal stage of development takes place. In human prenatal development, fetal dev ...
, however, there is evidence that methylation is increased during pregnancy and that it could be highly protective for the developing organism.
Enzymatic methylation of arsenic is a detoxification process; it can be methylated to methylarsenite, dimethylarsenite or trimethylarsenite, all of which are trivalent. The methylation is catalyzed by arsenic methyltransferase (AS3MT) in mammals, which transfers a methyl group on the cofactor S-adenomethionine (SAM) to arsenic (III). An orthologue of AS3MT is found in bacteria and is called CmArsM. This enzyme was tested in three states (ligand free, arsenic (III) bound and SAM bound). Arsenic (III) binding sites usually use thiol groups of cysteine residues. The catalysis involves thiolates of Cys72, Cys174, and Cys224. In an SN2 reaction, the positive charge on the SAM sulfur atom pulls the bonding electron from the carbon of the methyl group, which interacts with the arsenic lone pair to form an As−C bond, leaving SAH.
Excretion
In humans, the major route of excretion of most arsenic compounds is via theurine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excreted from the body through the urethra.
Cellular ...
. The biological half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of inorganic arsenic is about 4 days, but is slightly shorter following exposure to arsenate than to arsenite. The main metabolites excreted in the urine of humans exposed to inorganic arsenic are mono- and dimethylated arsenic acid
Arsenic acid or trihydrogen arsenate is the chemical compound with the formula . More descriptively written as , this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as ...
s, together with some unmetabolized inorganic arsenic.
The biotransformation of arsenic for excretion is primarily done through the nuclear factor erythroid 2 related factor 2 (Nrf2
Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the ''NFE2L2'' gene. NRF2 is a basic leucine zipper (bZIP) protein that may reg ...
) pathway. Under normal conditions the Nrf2 is bound to Kelch-like ECH associated protein 1 (Keap1
Kelch-like ECH-associated protein 1 is a protein that in humans is encoded by the ''Keap1'' gene.
Structure
Keap1 has four discrete protein domains. The N-terminal Broad complex, Tramtrack and Bric-à-Brac (BTB) domain contains the Cys151 res ...
) in its inactive form. With the uptake of arsenic within cells and the subsequent reactions that result in the production of reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS), the Nrf2 unbinds and becomes active. Keap1 has reactive thiol moieties that bind ROS or electrophilic arsenic species such as monomethylted arsenic (III) and induces the release of Nrf2 which then travels through the cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. ...
to the nucleus. The Nrf2 then activates antioxidant responsive element (ARE) as well as electrophilic responsive element (EpRE) both of which contribute in the increase of antioxidant proteins. Of particular note in these antioxidant proteins is heme oxygenase 1 ( O-1, NAD(P)H-quinone oxidoreductase 1 (NQO1), and γ-glutamylcysteine synthase (γGCS) which work in conjunction to reduce the oxidative species such as hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
to decrease the oxidative stress upon the cell. The increase in γGCS causes an increased production of arsenite triglutathionine (As(SG)3) an important adduct that is taken up by either multidrug associated protein 1 or 2 (MRP1
Multidrug resistance-associated protein 1 (MRP1) is a protein that in humans is encoded by the ''ABCC1'' gene.
Function
The protein encoded by this gene is a member of the superfamily of ATP-binding cassette (ABC) transporters. ABC proteins t ...
or MRP2) which removes the arsenic out of the cell and into bile for excretion. This adduct can also decompose back into inorganic arsenic.
Of particular note in the excretion of arsenic is the multiple methylation steps that take place which may increase the toxicity of arsenic due to MMeAsIII being a potent inhibitor of glutathione peroxidase, glutathione reductase, pyruvate dehydrogenase, and thioredoxin reductase.
Arsenic toxicity
Arsenic is a cause of mortality throughout the world; associated problems include heart, respiratory, gastrointestinal, liver, nervous and kidney diseases. Arsenic interferes with cellular longevity by allosteric inhibition of an essential metabolic enzymepyruvate dehydrogenase
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.
Pyruvate dehydrogenase is u ...
(PDH) complex, which catalyzes the oxidation of pyruvate to acetyl-CoA by NAD+. With the enzyme inhibited, the energy system of the cell is disrupted resulting in a cellular apoptosis episode. Biochemically, arsenic prevents use of thiamine resulting in a clinical picture resembling thiamine deficiency
Thiamine deficiency is a medical condition of low levels of thiamine (Vitamin B1). A severe and chronic form is known as beriberi. The two main types in adults are wet beriberi and dry beriberi. Wet beriberi affects the cardiovascular system, r ...
. Poisoning with arsenic can raise lactate levels and lead to lactic acidosis
Lactic acidosis is a medical condition characterized by a build-up of lactate (especially -lactate) in the body, with formation of an excessively low pH in the bloodstream. It is a form of metabolic acidosis, in which excessive acid accumulates d ...
.
Genotoxicity Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic su ...
involves inhibition of DNA repair and DNA methylation. The carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive subs ...
effect of arsenic arises from the oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal ...
induced by arsenic. Arsenic's high toxicity naturally led to the development of a variety of arsenic compounds as chemical weapon
A chemical weapon (CW) is a specialized munition that uses chemicals formulated to inflict death or harm on humans. According to the Organisation for the Prohibition of Chemical Weapons (OPCW), this can be any chemical compound intended as a ...
s, e.g. dimethylarsenic chloride. Some were employed as chemical warfare agent
A chemical weapon (CW) is a specialized munition that uses chemicals formulated to inflict death or harm on humans. According to the Organisation for the Prohibition of Chemical Weapons (OPCW), this can be any chemical compound intended as a ...
s, especially in World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
. This threat led to many studies on antidotes and an expanded knowledge of the interaction of arsenic compounds with living organisms. One result was the development of antidotes such as British anti-Lewisite
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is no ...
. Many such antidotes exploit the affinity of As(III) for thiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
s, which convert highly toxic organoarsenicals to less toxic derivatives. It is generally assumed that arsenates bind to cysteine residues in proteins.
By contrast, arsenic oxide is an approved and effective chemotherapeutic drug for the treatment of acute promyelocytic leukemia (APL).
Toxicity of pentavalent arsenicals
Due to its similar structure and properties, pentavalent arsenic metabolites are capable of replacing the phosphate group of many metabolic pathways. The replacement of phosphate by arsenate is initiated when arsenate reacts with glucose and gluconate in vitro. This reaction generates glucose-6-arsenate and 6-arsenogluconate, which act as analogs for glucose-6-phosphate and 6-phosphogluconate. At the substrate level, during glycolysis, glucose-6-arsenate binds as a substrate to glucose-6-phosphate dehydrogenase, and also inhibits hexokinase through negative feedback. Unlike the importance of phosphate in glycolysis, the presence of arsenate restricts the generation of ATP by forming an unstable anhydride product, through the reaction with D-glyceraldehyde-3-phosphate. The anhydride 1-arsenato-3-phospho-D-glycerate generated readily hydrolyzes due to the longer bond length of As-O compared to P-O. At the mitochondrial level, arsenate uncouples the synthesis of ATP by binding to ADP in the presence ofsuccinate
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological ro ...
, thus forming an unstable compound that ultimately results in a decrease of ATP net gain. Arsenite (III) metabolites, on the other hand, have limited effect on ATP production in red blood cells.
Toxicity of trivalent arsenicals
Enzymes and receptors that contain thiol or sulfhydryl functional groups are actively targeted by arsenite (III) metabolites. These sulfur-containing compounds are normally glutathione and theamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
cysteine. Arsenite derivatives generally have higher binding affinity compared to the arsenate metabolites. These bindings restrict activity of certain metabolic pathways. For example, pyruvate dehydrogenase (PDH) is inhibited when monomethylarsonous acid (MMAIII) targets the thiol group of the lipoic acid cofactor. PDH is a precursor of acetyl-CoA, thus the inhibition of PDH eventually limits the production of ATP in electron transport chain, as well as the production of gluconeogenesis intermediates.
Oxidative stress
Arsenic can cause oxidative stress through the formation ofreactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS), and reactive nitrogen species
Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•−) produced via the enzymatic activity of inducible nitric oxide synthase 2 ( NOS2) and NADPH oxidase respectivel ...
(RNS). Reactive oxygen species are produced by the enzyme NADPH oxidase
NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white ...
, which transfers electrons from NADPH to oxygen, synthesizing a superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
, which is a reactive free radical. This superoxide can react to form hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
and a reactive oxygen species. The enzyme NADPH oxidase
NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white ...
is able to generate more reactive oxygen species in the presence of arsenic, due to the subunit p22phax, which is responsible for the electron transfer, being upregulated by arsenic. The reactive oxygen species are capable of stressing the endoplasmic reticulum, which increases the amount of the unfolded protein response signals. This leads to inflammation, cell proliferation, and eventually to cell death. Another mechanism in which reactive oxygen species cause cell death would be through the cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is com ...
rearrangement, which affects the contractile proteins.
The reactive nitrogen species arise once the reactive oxygen species destroy the mitochondria. This leads to the formation of the reactive nitrogen species, which are responsible for damaging DNA in arsenic poisoning. Mitochondrial damage is known to cause the release of reactive nitrogen species, due to the reaction between superoxides and nitric oxide (NO). Nitric oxide (NO) is a part of cell regulation, including cellular metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
, growth, division and death. Nitric oxide (NO) reacts with reactive oxygen species to form peroxynitrite
Peroxynitrite (sometimes called peroxonitrite) is an ion with the formula ONOO−. It is a structural isomer of nitrate,
Preparation
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide:
:
It is prepared by the react ...
. In cases of chronic arsenic exposure, the nitric oxide levels are depleted, due to the superoxide reactions. The enzyme NO synthase (NOS) uses L-arginine to form nitric oxide, but this enzyme is inhibited by monomethylated arsenic (III) compounds.
DNA damage
Arsenic is reported to cause DNA modifications such asaneuploidy
Aneuploidy is the presence of an abnormal number of chromosomes in a cell, for example a human cell having 45 or 47 chromosomes instead of the usual 46. It does not include a difference of one or more complete sets of chromosomes. A cell with any ...
, micronuclei
Micronucleus is the name given to the small nucleus that forms whenever a chromosome or a fragment of a chromosome is not incorporated into one of the daughter nuclei during cell division. It usually is a sign of genotoxic events and chromosomal i ...
formation, chromosome abnormality
A chromosomal abnormality, chromosomal anomaly, chromosomal aberration, chromosomal mutation, or chromosomal disorder, is a missing, extra, or irregular portion of Chromosome, chromosomal DNA. These can occur in the form of numerical abnormalities ...
, deletion mutations, sister chromatid exchange
Sister chromatid exchange (SCE) is the exchange of genetic material between two identical sister chromatids.
It was first discovered by using the Giemsa staining method on one chromatid belonging to the sister chromatid complex before anaphase i ...
and crosslinking of DNA with proteins. It has been demonstrated that arsenic does not directly interact with DNA and it is considered a poor mutagen, but instead, it helps mutagenicity of other carcinogens
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive subs ...
. For instance, a synergistic increase in the mutagenic activity of arsenic with UV light has been observed in human and other mammalian cells after exposing the UV-treated cells to arsenic. A series of experimental observations suggest that the arsenic genotoxicity Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic su ...
is primarily linked to the generation of reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS) during its biotransformation. The ROS production is able to generate DNA adducts, DNA strand breaks, crosslinks and chromosomal aberrations. The oxidative damage is caused by modification of DNA nucleobases, in particular 8-oxoguanine
8-Oxoguanine (8-hydroxyguanine, 8-oxo-Gua, or OH8Gua) is one of the most common DNA lesions resulting from reactive oxygen species modifying guanine, and can result in a mismatched pairing with adenine resulting in G to T and C to A substitutions ...
(8-OHdG) which leads to G:C to T:A mutations. Inorganic arsenic can also cause DNA strand break even at low concentrations.
Inhibition of DNA repair
Inhibition ofDNA repair
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA da ...
processes is considered one of main mechanism of inorganic arsenic genotoxicity. Nucleotide excision repair (NER) and base excision repair
Base excision repair (BER) is a cellular mechanism, studied in the fields of biochemistry and genetics, that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from t ...
(BER) are the processes implicated in the repair of DNA base damage induced by ROS after arsenic exposure. In particular, the NER mechanism is the major pathway for repairing bulky distortions in DNA double helix, while the BER mechanism is mainly implicated in the repair of single strand breaks induced by ROS, but inorganic arsenic could also repress the BER mechanism.
Neurodegenerative mechanisms
Arsenic is highly detrimental to the innate and the adaptiveimmune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint ...
of the body. When the amount of unfolded and misfolded proteins in endoplasmic reticulum stress is excessive, the unfolded protein response
The unfolded protein response (UPR) is a cellular stress response related to the endoplasmic reticulum (ER) stress. It has been found to be conserved between all mammalian species, as well as yeast and worm organisms.
The UPR is activated in resp ...
(UPR) is activated to increase the activity of several receptors that are responsible the restoration of homeostasis. The inositol-requiring enzyme-1 (IRE1) and protein kinase RNA-like endoplasmic reticulum kinase (PERK) are two receptors that restrict the rate of translation. On the other hand, the unfolded proteins are corrected by the production of chaperones, which are induced by the activating transcription factor 6 (ATF6). If the number of erroneous proteins elevates, further mechanism is active which triggers apoptosis. Arsenic has evidentially shown to increase the activity of these protein sensors.
Immune dysfunction
Arsenic exposure in small children distorts the ratio ofT helper cell
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are consider ...
s ( CD4) to cytotoxic T cells (CD8
CD8 (cluster of differentiation 8) is a transmembrane glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). Along with the TCR, the CD8 co-receptor plays a role in T cell signaling and aiding with cytotoxic T cell-antigen int ...
), which are responsible for immunodepression.Vega, L. ''Environmental Health Risks''. Nova Science Publishers. pp157-159. In addition, arsenic also increases the number of inflammatory molecules being secreted through macrophages. The excess amount of granulocytes
Granulocytes are
cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear. They ha ...
and monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s lead to a chronic state of inflammation, which might result in cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
development.
Arsenic poisoning treatment
There are three molecules that serve as chelator agents that bond to arsenic. These three areBritish Anti-Lewisite
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is no ...
(BAL, Dimercaprol), succimer ( DMSA) and Unithiol ( DMPS).
When these agents chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
inorganic arsenic, it is converted into an organic form of arsenic because it is bound to the organic chelating agent. The sulfur atoms of the thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
groups are the site of interaction with arsenic. This is because the thiol groups are nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
while the arsenic atoms are electrophilic. Once bound to the chelating agent the molecules can be excreted, and therefore free inorganic arsenic atoms are removed from the body.
Other chelating agents can be used, but may cause more side effects than British Anti-Lewisite (BAL, Dimercaprol), succimer ( DMSA) and ( DMPS). DMPS and DMSA also have a higher therapeutic index
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes ...
than BAL.
These drugs are efficient for acute poisoning of arsenic, which refers to the instantaneous effects caused by arsenic poisoning. For example, headaches, vomiting or sweating are some of the common examples of an instantaneous effect. In comparison, chronic poisonous effects arise later on, and unexpectedly such as organ damage. Usually it is too late to prevent them once they appear. Therefore, action should be taken as soon as acute poisonous effects arise.
See also
* Arsenic compounds *Extremophile
An extremophile (from Latin ' meaning "extreme" and Greek ' () meaning "love") is an organism that is able to live (or in some cases thrive) in extreme environments, i.e. environments that make survival challenging such as due to extreme temper ...
* Geomicrobiology
Geomicrobiology is the scientific field at the intersection of geology and microbiology and is a major subfield of geobiology. It concerns the role of microbes on geological and geochemical processes and effects of minerals and metals to microb ...
* Hypothetical types of biochemistry
Hypothetical types of biochemistry are forms of biochemistry agreed to be scientifically viable but not proven to exist at this time. The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabo ...
* Organoarsenic chemistry
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungic ...
References
{{DEFAULTSORT:Arsenic biochemistry Arsenic Biology and pharmacology of chemical elements