Aralkylamine N-acetyltransferase on:
[Wikipedia]
[Google]
[Amazon]
Aralkylamine ''N''-acetyltransferase (AANAT) (), also known as arylalkylamine ''N''-acetyltransferase or serotonin ''N''-acetyltransferase (SNAT), is an
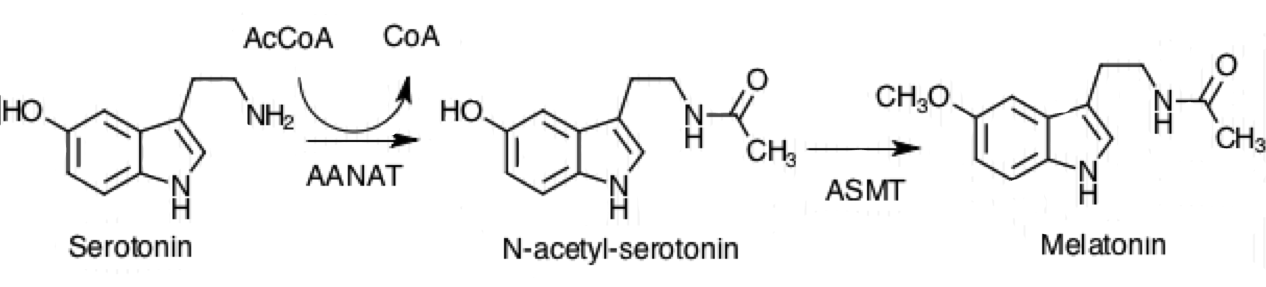 In the biosynthesis of melatonin, N-acetylserotonin is further
In the biosynthesis of melatonin, N-acetylserotonin is further
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb '' AANAT gene'' containing four exons, located on chromosome 17
Chromosome 17 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 17 spans more than 83 million base pairs (the building material of DNA) and represents between 2.5 and 3% of the total D ...
q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of ''N''-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosomal abnormality. Although polygenic disorders ...
such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.
Nomenclature
Thesystematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivial ...
of this enzyme class is acetyl-CoA:2-arylethylamine N-acetyltransferase. Other names in common use include:
* AANAT
* Arylalkylamine N-acetyltransferase
* Melatonin rhythm enzyme
* Serotonin acetylase
* Serotonin acetyltransferase
* Serotonin N-acetyltransferase
The officially accepted name is aralkylamine N-acetyltransferase.
Function and mechanism
Tissue distribution
The AANAT mRNA transcript is mainly expressed in thecentral nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all p ...
(CNS). It is detectable at low levels in several brain
A brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It is located in the head, usually close to the sensory organs for senses such as vision. It is the most complex organ in a ve ...
regions including the pituitary gland
In vertebrate anatomy, the pituitary gland, or hypophysis, is an endocrine gland, about the size of a chickpea and weighing, on average, in humans. It is a protrusion off the bottom of the hypothalamus at the base of the brain. The ...
as well as in the retina
The retina (from la, rete "net") is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then ...
. It is most highly abundant in the pineal gland which is the site of melatonin synthesis. Brain and pituitary AANAT may be involved in the modulation of serotonin-dependent aspects of human behavior and pituitary function.
Physiological function
In thepinealocyte
Pinealocytes are the main cells contained in the pineal gland, located behind the third ventricle and between the two hemispheres of the brain. The primary function of the pinealocytes is the secretion of the hormone melatonin, important in th ...
cells of the pineal gland, aralkylamine N-acetyltransferase is involved in the conversion of serotonin to melatonin. It is the penultimate enzyme in the melatonin synthesis controlling the night/day rhythm in melatonin production in the vertebrate pineal gland. Melatonin is essential for seasonal reproduction, modulates the function of the circadian clock in the suprachiasmatic nucleus, and influences activity and sleep. Due to its important role in circadian rhythm, AANAT is subjected to extensive regulation that is responsive to light exposure (see Regulation). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and mood disorders.
The chemical reactions catalyzed by AANAT
The primarychemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
that is catalyzed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
by aralkylamine N-acetyltransferase uses two substrates, acetyl-CoA and serotonin. AANAT catalyzes the transfer of the acetyl group of Acetyl-CoA to the primary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
of serotonin, thereby producing CoA and N-acetylserotonin. In humans, other endogenous substrates of the enzyme include specific trace amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to th ...
neuromodulators, namely phenethylamine, tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood ...
, and tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ...
, in turn forming N-acetylphenethylamine, N-acetyltyramine, and N-acetyltryptamine.
 In the biosynthesis of melatonin, N-acetylserotonin is further
In the biosynthesis of melatonin, N-acetylserotonin is further methylated
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These ...
by another enzyme, N-acetylserotonin O-methyltransferase (ASMT) to generate melatonin. The N-acetyltransferase reaction has been suggested to be the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
, and thus Serotonin N-acetyltransferase has emerged as a target for inhibitor design (see below).
AANAT obeys an ordered ternary-complex mechanism. The substrates bind sequentially (ordered) with acetyl-CoA binding to the free enzyme followed by the binding of serotonin to form the ternary complex. After the transfer of the acetyl group has occurred, the products are orderly released with N-acetyl-serotonin first and CoA last.
Structure
Arylkylamine N-acetyltransferase is amonomeric
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
polypeptide with a length of 207 amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
residues, and with a molecular weight of 23,344 daltons. The secondary structure consists of alpha helices
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
and beta sheets
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gen ...
. It is 28 percent helical (10 helices; 60 residues) and 23 percent beta sheet (9 strands; 48 residues). This family shares four conserved sequence motifs designated A-D. Motif B serves as the location of the serotonin binding slot. The structure was determined by X-ray diffraction.
Several structures
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
have been solved for this class of enzymes, with PDB accession codes , , , and //.
Aralkylamine N-acetyltransferase has also been crystallized in complex with 14-3-3ζ from the 14-3-3 protein family, with the PDB accession code .
The GNAT superfamily
Aralkylamine N-acetyltransferase belongs to the GCN5-related N-acetyltransferase (GNAT) superfamily which consists 10,000 acetyltransferases, named so because of their sequence homology to a class of eukaryotictranscription factors
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The fun ...
, therein the yeast GCN5. Other well-studied members of the superfamily are glucosamine-6-phosphate N-acetyltransferase and histone acetyltransferases
Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-''N''-acetyllysine. DNA is wrapped around histones, and, by transferring an ...
.
All members of this superfamily has a structurally conserved fold consisting of an N-terminal strand followed by two helices, three antiparallel β-strands, followed by a ‘‘signature’’ central helix, a fifth β-strand, a fourth α-helix and a final β-strand. These elements are nearly universally conserved in spite of poor pairwise identity in sequence alignments.
Regulation
Regulation of AANAT varies between species. In some, AANAT levels oscillate dramatically between light and dark periods, and thus control melatonin synthesis. In others, rhythm is regulated primarily on the protein level. One example is in rodents, where AANAT mRNA levels increase more than 100-fold in dark periods. In other species,cyclic AMP
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal tra ...
plays an important part in inhibition of proteolytic degradation
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, ...
of AANAT, elevating protein levels at night. Experiments using human AANAT expressed in a 1E7 cell line show an ∼8-fold increase in enzyme activity upon exposure to forskolin
Forskolin (coleonol) is a labdane diterpene produced by the plant ''Coleus barbatus'' (Blue Spur Flower). Other names include pashanabhedi, Indian coleus, makandi, HL-362, mao hou qiao rui hua. As with other members of the large diterpene class of ...
.
Dynamic degradation of AANAT mRNA has proven essential to the circadian action of the enzyme. The 3’UTR sequences have importance with regards to the rhythmic degradation of AANAT mRNA in some species. In rodents, various hnRNPs maintain dynamic degradation of AANAT mRNA. In other species, such as ungulates and primates, the stable AANAT mRNAs with a shorter 3’UTR is suspected not to be under control of the hnRNPs that bind and direct degradation of AANAT mRNA in rodents.
Exposure to light induces signals to travel from retinal cells, ultimately causing a drop in norepinephrine
Norepinephrine (NE), also called noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and body as both a hormone and neurotransmitter. The name "noradrenaline" (from Latin '' ad' ...
stimulation of the pineal gland. This, in turn, leads to a signaling cascade, resulting in Protein Kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
phosphorylation of two key Ser and Thr residues of serotonin N-acetyltransferase. Phosphorylation of these residues causes changes in catalytic activity through recruitment and interaction with 14-3-3 proteins, specifically 14-3-3ζ.
Another protein which interacts and regulates AANAT activity is protein kinase C. Protein kinase C acts, like protein kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
, on threonine and serine residues, enhancing the stability and enzymatic activity of AANAT.
Inhibition of the acetyl-CoA-binding to the catalytic site through the formation and cleavage of intramolecular disulfide bonds has been suggested to be a mechanism of regulation. Formation of a disulfide bond between two cystein residues within the protein closes the hydrophobic funnel of the catalytic site, and thus acts as an on/off switch for catalytic activity. It is not yet certain if this mechanism is present in ''in vivo'' cells through the regulation of intracellular redox conditions, but it is suggested that glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
(GSH) could be an ''in vivo'' regulator of the formation and cleavage of these disulfide bonds.
AANAT inhibitors and clinical relevance
Inhibitors of AANAT may eventually lead to development of a drug that would be useful in circadian biology research and in the treatment of sleep andmood disorders
A mood disorder, also known as an affective disorder, is any of a group of conditions of mental and behavioral disorder where a disturbance in the person's mood is the main underlying feature. The classification is in the '' Diagnostic and St ...
. Synthetic inhibitors of the enzyme have been discovered. However, no AANAT inhibitor with potent ''in vivo'' activity has been reported. Up to now, five classes of AANAT inhibitors have been described in the literature. Below are the five classes:
Melatonin derivatives
Since it was reported that melatonin is acompetitive inhibitor
Competitive inhibition is interruption of a chemical pathway owing to one chemical substance inhibiting the effect of another by competing with it for binding or bonding. Any metabolic or chemical messenger system can potentially be affected b ...
of AANAT, this neurotransmitter seems to exert an autoregulatory control on its own biosynthesis. Thus, loose structural analogues of the indolamine hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
were evaluated on AANAT, and moderate inhibitors were discovered.
Peptidic inhibitors
Peptide combinatorial libraries of tri-, tetra-, and pentapeptides with various amino acid compositions were screened as potential sources of inhibitors, to see if it serves as either pure or mixed competitive inhibitor for the hAANAT enzyme.Molecular modeling
Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials scien ...
and structure-activity relationship studies made it possible to pinpoint the amino acid residue of the pentapeptide inhibitor S 34461 that interacts with the cosubstrate-binding site.
Bisubstrate analogs
It is suggested that AANAT catalyzes the transfer of an acetyl group from acetyl-CoA to serotonin, with the involvement of an intermediateternary complex
A ternary complex is a protein complex containing three different molecules that are bound together. In structural biology, ''ternary complex'' can also be used to describe a crystal containing a protein with two small molecules bound, for example ...
, to produce N-acetylserotonin. Based on this mechanism, it might be expected that a bisubstrate analog inhibitor, derived from the tethering of indole and CoASH parts, could potentially mimic the ternary complex and exert strong inhibition of AANAT. The first bisubstrate analog (1), which links tryptamine
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ...
and CoA via an acetyl bridge, was synthesized by Khalil and Cole, and shown to be a very potent and specific AANAT inhibitor.
N-Haloacetylated derivatives
AANAT has shown that it also has a secondary alkyltransferase activity as well as acetyltransferase activity. N-Haloacetyltryptamines were developed and serve as substrates of AANAT alkyltransferase and are also potent (low micromolar) ''in vitro'' inhibitors against AANAT acetyltransferase activity. AANAT catalyzes reaction between N-bromoacetyltryptamine (BAT) and reduced CoA, resulting a tight-binding bisubstrate analog inhibitor. The first synthesized cell-permeable inhibitor of AANAT N-bromoacetyltryptamine was studied further on melatonin secretion from rat and pig pineal glands. New N-halogenoacetyl derivatives leading to a strongin situ
''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in ...
inhibition of AANAT. The concept behind the mechanism of action of these precursors was studied by following the biosynthesis of the inhibitor from tritiated-BAT in a living cell.
Rhodanine-based compounds
The first druglike and selective inhibitors of AANAT has been identified. Lawrence M. Szewczuk ''et al.'' have virtually screened more than a million compounds by 3D high-throughput docking into the active site ofX-ray structure
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
for AANAT, and then tested 241 compounds as inhibitors. One compound class which containing a rhodanine scaffold has shown low micromolar competitive inhibition against acetyl-CoA and proved to be effective in blocking melatonin production in pineal cells.
The recent study about inhibitor of AANAT has described the discovery of a new class of nonpeptidic AANAT inhibitors based on a 2,2′-bithienyl scaffold.
See also
* AcetyltransferaseReferences
Further reading
* * *External links
* * * {{NLM content EC 2.3.1 Enzymes of known structure Circadian rhythm