Antimicrobial peptides on:
[Wikipedia]
[Google]
[Amazon]
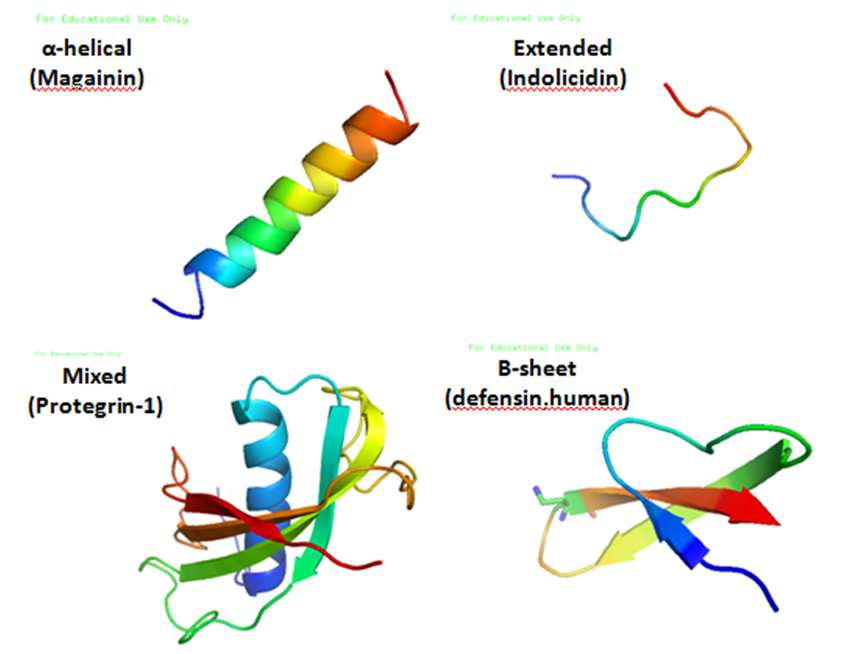 Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between
 Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are generally between 12 and 50 amino acids. These peptides include two or more positively charged residues provided by arginine, lysine or, in acidic environments,
Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are generally between 12 and 50 amino acids. These peptides include two or more positively charged residues provided by arginine, lysine or, in acidic environments,
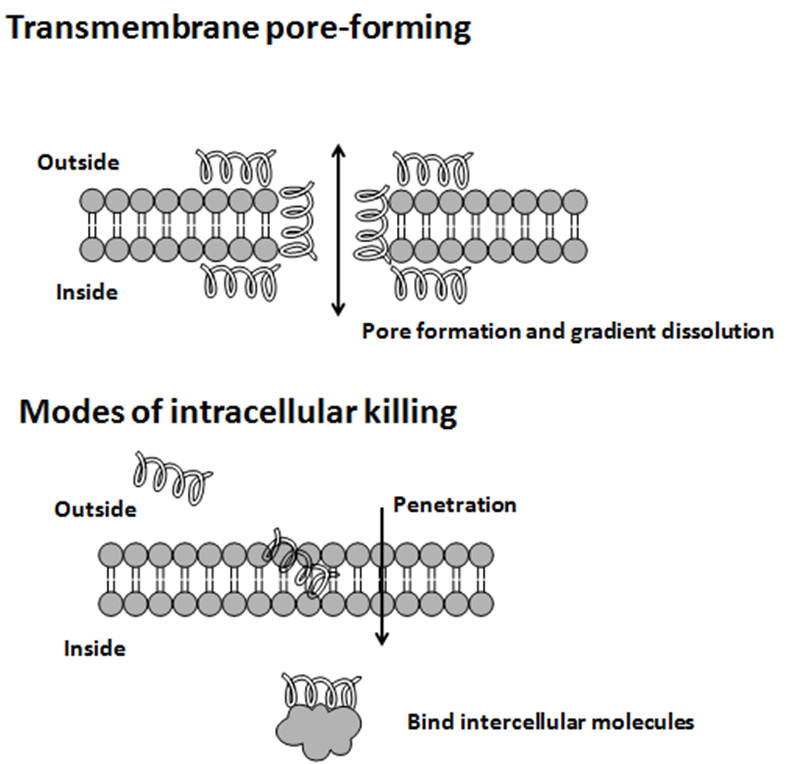 The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The
The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The
 Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular,
Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular,
and LAMP (Linking AMPs). The Antimicrobial peptide databases may be divided into two categories on the basis of the source of peptides it contains, as specific databases and general databases. These databases have various tools for antimicrobial peptides analysis and prediction. For example, the APD has a widely used calculation interface. It also provides links to many other tools. CAMP contains AMP prediction, feature calculator, BLAST search, ClustalW, VAST, PRATT, Helical wheel etc. In addition, ADAM allows users to search or browse through AMP sequence-structure relationships. Antimicrobial peptides often encompass a wide range of categories such as antifungal, antibacterial, and antituberculosis peptides. : Provides an online platform for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. is an online resource that addresses various topics such as annotations of antimicrobial peptides (AMPs) including sequence information, antimicrobial activities, post-translational modifications (PTMs), structural visualization, antimicrobial potency, target species with minimum inhibitory concentration (MIC), physicochemical properties, or AMP–protein interactions. Tools such as PeptideRanker, PeptideLocator, and AntiMPmod allow for the prediction of antimicrobial peptides while others have been developed to predict antifungal and anti-Tuberculosis activities.
ADAM (A Database of Anti-Microbial peptides)
at ntou.edu.tw
AntiFP
Prediction of antifungal peptides
AntiMPmod
Prediction of antimicrobial potential of modified peptides *
AntiTbPred
Prediction of anti-tuberculosis peptides
Antimicrobial Peptide Database
at University of Nebraska Medical Center
Antimicrobial Peptide Scanner
Deep Learning based AMP prediction server
AntiTbPdb
Anti Tubercular Peptide Database
BioPD
at Peking University Health Science Center
CAMP:Collection of Anti-Microbial Peptides
at National Institute for Research in Reproductive Health (NIRRH)
DBAASP
- Database of Antimicrobial Activity and Structure of Peptides]
LAMP
at Fudan University
PeptideLocator
Prediction of functional peptides, including antimicrobial peptides, in a protein sequence
PeptideRanker
Bioactive peptide, including antimicrobial peptide, prediction
modlAMP
Python package for computational work with antimicrobial peptides, including sequence handling, -design, -prediction, descriptor calculation and plotting {{DEFAULTSORT:Antimicrobial Peptides Antimicrobial peptides, Immunology Immune system Peripheral membrane proteins Insect immunity
 Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic
A prokaryote () is a single-celled organism that lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek πρό (, 'before') and κάρυον (, 'nut' or 'kernel').Campbell, N. "Biology:Concepts & Connec ...
and eukaryotic
Eukaryotes () are organisms whose Cell (biology), cells have a cell nucleus, nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the ...
cells that may represent targets for antimicrobial peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
s. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative
The gram (originally gramme; SI unit symbol g) is a unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram.
Originally defined as of 1795 as "the absolute weight of a volume of pure water equal to th ...
and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels Transmembrane channels, also called membrane channels, are pores within a lipid bilayer. The channels can be formed by protein complexes that run across the membrane or by peptides. They may cross the cell membrane, connecting the cytosol, or cytop ...
, and may also have the ability to enhance immunity by functioning as immunomodulator
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
s.
Structure
histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
, and a large proportion (generally >50%) of hydrophobic residues. The secondary structures of these molecules follow 4 themes, including i) α-helical, ii) β-stranded due to the presence of 2 or more disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s, iii) β-hairpin or loop due to the presence of a single disulfide bond and/or cyclization of the peptide chain, and iv) extended. Many of these peptides are unstructured in free solution, and fold into their final configuration upon partitioning into biological membranes. It contains hydrophilic amino acid residues aligned along one side and hydrophobic amino acid residues aligned along the opposite side of a helical molecule. This amphipathicity of the antimicrobial peptides allows them to partition into the membrane lipid bilayer. The ability to associate with membranes is a definitive feature of antimicrobial peptides, although membrane permeabilization is not necessary. These peptides have a variety of antimicrobial activities ranging from membrane permeabilization to action on a range of cytoplasmic targets.
Activities
 The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The
The modes of action by which antimicrobial peptides kill microbes are varied, and may differ for different bacterial species. Some antimicrobial peptides kill both bacteria and fungi, e.g., psoriasin kills ''E. coli'' and several filamentous fungi. The cytoplasmic membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (th ...
is a frequent target, but peptides may also interfere with DNA and protein synthesis, protein folding, and cell wall synthesis. The initial contact between the peptide and the target organism is electrostatic, as most bacterial surfaces are anionic, or hydrophobic, such as in the antimicrobial peptide Piscidin. Their amino acid composition, amphipathicity, cationic charge and size allow them to attach to and insert into membrane bilayers to form pores by ‘barrel-stave’, ‘carpet’ or ‘toroidal-pore’ mechanisms. Alternately, they may penetrate into the cell to bind intracellular molecules which are crucial to cell living.
Intracellular binding models includes inhibition of cell wall synthesis, alteration of the cytoplasmic membrane, activation of autolysin, inhibition of DNA, RNA, and protein synthesis, and inhibition of certain enzymes. However, in many cases, the exact mechanism of killing is not known. One emerging technique for the study of such mechanisms is dual polarisation interferometry
Dual-polarization interferometry (DPI) is an analytical technique that probes molecular layers adsorbed to the surface of a waveguide using the evanescent wave of a laser beam. It is used to measure the conformational change in proteins, or othe ...
. In contrast to many conventional antibiotics these peptides appear to be bactericidal instead of bacteriostatic
A bacteriostatic agent or bacteriostat, abbreviated Bstatic, is a biological or chemical agent that stops bacteria from reproducing, while not necessarily killing them otherwise. Depending on their application, bacteriostatic antibiotics, disinfect ...
. In general the antimicrobial activity of these peptides is determined by measuring the minimal inhibitory concentration (MIC), which is the lowest concentration of drug that inhibits bacterial growth.
AMPs can possess multiple activities including anti-gram-positive bacterial, anti-gram-negative bacterial, anti-fungal, anti-viral, anti-parasitic, and anti cancer activities. A big AMP functional analysis indicates that among all AMP activities, amphipathicity and charge, two major properties of AMPs, best distinguish between AMPs with and without anti-gram-negative bacterial activities. This implies that being AMPs with anti-gram-negative bacterial activities may prefer or even require strong amphipathicity and net positive charge.
Immunomodulation
In addition to killing bacteria directly they have been demonstrated to have a number ofimmunomodulator
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
y functions that may be involved in the clearance of infection, including the ability to alter host gene expression, act as chemokines and/or induce chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or signaling proteins secreted by cells that induce directional movement of leukocytes, as well as other cell types, including endothelial and epithelial cells. In additio ...
production, inhibiting lipopolysaccharide
Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide that are bacterial toxins. They are composed of an O-antigen, an outer core, and an inner core all joined by a covalent bond, and are found in the outer ...
induced pro-inflammatory cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
production, promoting wound healing, and modulating the responses of dendritic cells and cells of the adaptive immune response. Animal models indicate that host defense peptides are crucial for both prevention and clearance of infection. It appears as though many peptides initially isolated as and termed "antimicrobial peptides" have been shown to have more significant alternative functions in vivo (e.g. hepcidin
). Dusquetide for example is an immunomodulator that acts through p62, a protein involved in toll like receptor based signalling of infection. The peptide is being examined in a Phase III clinical trial by Soligenix (SGNX) to ascertain if it can assist in repair of radiation-induced damage to oral mucosa arising during cancer radiotherapy of the head and neck.
Mechanisms of action
 Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular,
Antimicrobial peptides generally have a net positive charge, allowing them to interact with the negatively charged molecules exposed on bacteria and cancer cell surfaces, such as phospholipid phosphatidylserine, O-glycosylated mucins, sialylated gangliosides, and heparin sulfates. The mechanism of action of these peptides varies widely but can be simplified into two categories: membranolytic and non-membranolytic antimicrobial peptides. The disruption of membranes by membranolytic antimicrobial peptides can be described by four models:
* toroidal model
* disordered toroidal-pore model
* carpet model
* barrel stave model
Although the specifics of each mechanism differ, all propose peptide-induced membrane rupture, allowing cytoplasmic leakage that ultimately leads to death.
Recent work has painted a more complex picture of antimicrobial peptide activity. The non-membranolytic antimicrobial peptides may also function as metabolic inhibitors, directly interacting with DNA, RNA, protein synthesis, and inhibitors of cell wall synthesis or septum formation. They are also known to cause ribosomal aggregation and delocalize membrane proteins.
Adding a further layer of complexity, many natural antimicrobial peptides possess weak bactericidal activity. Rather than directly inhibit bacterial growth, they are now known to act in concert with the host immune system through mechanisms including chemokine induction, histamine release, and angiogenesis modulation. These immunomodulatory effects have only recently begun to receive attention.
Several methods have been used to determine the mechanisms of antimicrobial peptide activity. In particular, solid-state NMR
Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic pa ...
studies have provided an atomic-level resolution explanation of membrane disruption by antimicrobial peptides. In more recent years, X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
has been used to delineate in atomic detail how the family of plant defensins rupture membranes by identifying key phospholipids in the cell membranes of the pathogen. Human defensins have been thought to act through a similar mechanism, targeting cell membrane lipids as part of their function. In fact human beta-defensin 2 have now been shown to kill the pathogenic fungi ''Candida albicans
''Candida albicans'' is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also survive outside the human body. It is detected in the gastrointestinal tract and mouth in 40–60% of healthy adults. It is usu ...
'' through interactions with specific phospholipids. From the computational point of view, the molecular dynamics simulations can shed light in the molecular mechanism and the specific peptide interactions with lipids, ions and solvent.
Therapeutic research and use
Antimicrobial peptides have been used as therapeutic agents; their use is generally limited to intravenous administration or topical applications due to their short half-lives. As of January 2018 the following antimicrobial peptides were in clinical use: * Bacitracin for pneumonia, topical * Boceprevir, Hepatitis C (oral, cyclic peptide) * Dalbavancin, bacterial infections, IV *Daptomycin
Daptomycin, sold under the brand name Cubicin among others, is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms.
Daptomycin was removed from the World Health Organiza ...
, bacterial infections, IV
* Enfuvirtide
Enfuvirtide ( INN) is an HIV fusion inhibitor, the first of a class of antiretroviral drugs used in combination therapy for the treatment of HIV-1 infection. It is marketed under the trade name Fuzeon (Roche).
Structural formula
Enfuvirtide i ...
, HIV, subcutaneous injection
* Oritavancin, bacterial infections, IV
* Teicoplanin, bacterial infections, IV
* Telaprevir
Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as ...
, Hepatitis C, oral cyclic peptide
* Telavancin, bacterial infection, IV
* Vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is recommended intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, ...
, bacterial infection, IV.
* Guavanin 2, bacterial infection against Gram-positive and Gram-negative also.
Activity beyond antibacterial functions
AMPs have been observed having functions other than bacterial and fungal killing. These activities include antiviral effects, but also roles in host defence such as anticancer functions and roles in neurology. This has led to a movement for re-branding AMPs as "Host-defence peptides" to encompass the broad scope of activities AMPs can have.Anticancer properties
Some cecropins (e.g. cecropin A, and cecropin B) have anticancer properties and are called anticancer peptides (ACPs). Hybrid ACPs based on Cecropin A have been studied for anticancer properties. The fruit fly Defensin prevents tumour growth, suspected to bind to tumour cells owing to cell membrane modifications common to most cancer cells, such asphosphatidylserine
Phosphatidylserine (abbreviated Ptd-L-Ser or PS) is a phospholipid and is a component of the cell membrane. It plays a key role in cell cycle signaling, specifically in relation to apoptosis. It is a key pathway for viruses to enter cells via ap ...
exposure.
Antibiofilm properties
Cecropin A can destroy planktonic and sessilebiofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular ...
-forming uropathogenic ''E. coli'' (UPEC) cells, either alone or when combined with the antibiotic nalidixic acid
Nalidixic acid (tradenames Nevigramon, NegGram, Wintomylon and WIN 18,320) is the first of the synthetic quinolone antibiotics.
In a technical sense, it is a naphthyridone, not a quinolone: its ring structure is a 1,8-naphthyridine nucleus that ...
, synergistically clearing infection in vivo (in the insect host ''Galleria mellonella
''Galleria mellonella'', the greater wax moth or honeycomb moth, is a moth of the family Pyralidae. ''G. mellonella'' is found throughout the world. It is one of two species of wax moths, with the other being the lesser wax moth. ''G. mellonella' ...
'') without off-target cytotoxicity. The multi-target mechanism of action involves outer membrane permeabilization followed by biofilm disruption triggered by the inhibition of efflux pump activity and interactions with extracellular and intracellular nucleic acids.
Other research
Recently there has been some research to identify potential antimicrobial peptides from prokaryotes, aquatic organisms such as fish, and shellfish, and monotremes such as echidnas.Selectivity
In the competition of bacterial cells and host cells with the antimicrobial peptides, antimicrobial peptides will preferentially interact with the bacterial cell to the mammalian cells, which enables them to kill microorganisms without being significantly toxic to mammalian cells. Selectivity is a very important feature of the antimicrobial peptides and it can guarantee their function as antibiotics in host defense systems. With regard tocancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
cells, they themselves also secrete human antimicrobial peptides including defensin, and in some cases, they are reported to be more resistant than the surrounding normal cells.
Therefore, we cannot conclude that selectivity is always high against cancer cells.
Factors
There are some factors that are closely related to the selectivity property of antimicrobial peptides, among which the cationic property contributes most. Since the surface of the bacterial membranes is more negatively charged than mammalian cells, antimicrobial peptides will show different affinities towards the bacterial membranes and mammalian cell membranes. In addition, there are also other factors that will affect the selectivity. It's well known thatcholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell mem ...
is normally widely distributed in the mammalian cell membranes as a membrane stabilizing agents but absent in bacterial cell membranes; and the presence of these cholesterols will also generally reduce the activities of the antimicrobial peptides, due either to stabilization of the lipid bilayer or to interactions between cholesterol and the peptide. So the cholesterol in mammalian cells will protect the cells from attack by the antimicrobial peptides.
Besides, the transmembrane potential is well known to affect peptide-lipid interactions. There's an inside-negative transmembrane potential existing from the outer leaflet to the inner leaflet of the cell membranes and this inside-negative transmembrane potential will facilitate membrane permeabilization probably by facilitating the insertion of positively charged peptides into membranes. By comparison, the transmembrane potential of bacterial cells is more negative than that of normal mammalian cells, so bacterial membrane will be prone to be attacked by the positively charged antimicrobial peptides.
Similarly, it is also believed that increasing ionic strength, which in general reduces the activity of most antimicrobial peptides, contributes partially to the selectivity of the antimicrobial peptides by weakening the electrostatic interactions
Electrostatics is a branch of physics that studies electric charges at Rest (physics), rest (static electricity).
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles af ...
required for the initial interaction.
Mechanism
The cell membranes of bacteria are rich in acidicphospholipids
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids t ...
, such as phosphatidylglycerol and cardiolipin. These phospholipid headgroups are heavily negatively charged. Therefore, the outmost leaflets of the bilayer which is exposed to the outside of the bacterial membranes are more attractive to the attack of the positively charged antimicrobial peptides. So the interaction between the positive charges of antimicrobial peptides and the negatively charged bacterial membranes is mainly the electrostatic interactions, which is the major driving force for cellular association. In addition, since antimicrobial peptides form structures with a positively charged face as well as a hydrophobic face, there are also some hydrophobic interactions between the hydrophobic regions of the antimicrobial peptides and the zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
ic phospholipids (electrically neutral) surface of the bacterial membranes, which act only as a minor effect in this case.
In contrast, the outer part of the membranes of plants and mammals is mainly composed of lipids without any net charges since most of the lipids with negatively charged headgroups are principally sequestered into the inner leaflet of the plasma membranes. Thus in the case of mammalian cells, the outer surfaces of the membranes are usually made of zwitterionic phosphatidylcholine
Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup.
They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybea ...
and sphingomyelin
Sphingomyelin (SPH, ˌsfɪŋɡoˈmaɪəlɪn) is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a phosp ...
, even though a small portion of the membrane's outer surfaces contain some negatively charged gangliosides
A ganglioside is a molecule composed of a glycosphingolipid (ceramide and oligosaccharide) with one or more sialic acids (e.g. ''N''-acetylneuraminic acid, NANA) linked on the sugar chain. NeuNAc, an acetylated derivative of the carbohydrate sial ...
. Therefore, the hydrophobic interaction between the hydrophobic face of amphipathic antimicrobial peptides and the zwitterionic phospholipids on the cell surface of mammalian cell membranes plays a major role in the formation of peptide-cell binding. However, the hydrophobic interaction is relatively weak when compared to the electrostatic interaction, thus, the antimicrobial peptides will preferentially interact with bacterial membranes.
Dual polarisation interferometry
Dual-polarization interferometry (DPI) is an analytical technique that probes molecular layers adsorbed to the surface of a waveguide using the evanescent wave of a laser beam. It is used to measure the conformational change in proteins, or othe ...
has been used ''in vitro'' to study and quantify the association to headgroup, insertion into the bilayer, pore formation and eventual disruption of the membrane.
Control
A lot of effort has been put into controlling cell selectivity. For example, attempts have been made to modify and optimize the physicochemical parameters of the peptides to control the selectivities, including net charge, helicity, hydrophobicity per residue (H), hydrophobic moment (μ) and the angle subtended by the positively charged polar helix face (Φ). Other mechanisms like the introduction of D- amino acids and fluorinated amino acids in the hydrophobic phase are believed to break the secondary structure and thus reduce hydrophobic interaction with mammalian cells. It has also been found that Pro→Nlys substitution in Pro-containing β-turn antimicrobial peptides was a promising strategy for the design of new small bacterial cell-selective antimicrobial peptides with intracellular mechanisms of action. It has been suggested that direct attachment ofmagainin
The magainins are a class of antimicrobial peptides found in the African clawed frog (''Xenopus laevis''). The peptides are cationic, generally lack a stable conformation in water but form amphipathic α-helix in membranes; their mechanism agains ...
to the substrate surface decreased nonspecific cell binding and led to improved detection limit for bacterial cells such as '' Salmonella'' and '' E. coli''.
Bacterial resistance
Bacteria use various resistance strategies to avoid antimicrobial peptide killing. Some microorganisms alter net surface charges. '' Staphylococcus aureus'' transports D-alanine from the cytoplasm to the surface teichoic acid which reduces the net negative charge by introducing basic amino groups. ''S. aureus'' also modifies its anionic membranes via MprF with L-lysine, increasing the positive net charge. The interaction of antimicrobial peptides with membrane targets can be limited by capsule polysaccharide of ''Klebsiella pneumoniae''. Alterations occur in Lipid A. ''Salmonella'' species reduce the fluidity of their outer membrane by increasing hydrophobic interactions between an increased number of Lipid A acyl tails by adding myristate to Lipid A with 2-hydroxymyristate and forming hepta-acylated Lipid A by adding palmitate. The increased hydrophobic moment is thought to retard or abolish antimicrobial peptide insertion and pore formation. The residues undergo alteration in membrane proteins. In some Gram-negative bacteria, alteration in the production of outer membrane proteins correlates with resistance to killing by antimicrobial peptides. Non-typeable ''Hemophilus influenzae
''Haemophilus influenzae'' (formerly called Pfeiffer's bacillus or ''Bacillus influenzae'') is a Gram-negative, non-motile, coccobacillary, facultatively anaerobic, capnophilic pathogenic bacterium of the family Pasteurellaceae. The bacter ...
'' transports AMPs into the interior of the cell, where they are degraded. Furthermore, ''H. influenzae'' remodels its membranes to make it appear as if the bacterium has already been successfully attacked by AMPs, protecting it from being attacked by more AMPs. ATP-binding cassette transporters import antimicrobial peptides and the resistance-nodulation cell-division efflux pump exports antimicrobial peptides. Both transporters have been associated with antimicrobial peptide resistance. Bacteria produce proteolytic enzymes, which may degrade antimicrobial peptides leading to their resistance. Outer membrane vesicles produced by Gram-negative bacteria bind the antimicrobial peptides and sequester them away from the cells, thereby protecting the cells. The outer membrane vesicles are also known to contain various proteases, peptidases and other lytic enzymes, which may have a role in degrading the extracellular peptide and nucleic acid molecules, which if allowed to reach to the bacterial cells may be dangerous for the cells. Cyclic-di-GMP signaling had also been involved in the regulation of antimicrobial peptide
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for a ...
resistance in ''Pseudomonas aeruginosa
''Pseudomonas aeruginosa'' is a common encapsulated, gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, ''P. aerug ...
''
While these examples show that resistance can evolve naturally, there is increasing concern that using pharmaceutical copies of antimicrobial peptides can make resistance happen more often and faster. In some cases, resistance to these peptides used as a pharmaceutical to treat medical problems can lead to resistance, not only to the medical application of the peptides, but to the physiological function of those peptides.
The ‘Trojan Horse’ approach to solving this problem capitalizes on the innate need for iron by pathogens. “Smuggling” antimicrobials into the pathogen is accomplished by linking them to siderophores for transport. While simple in concept, it has taken many decades of work to accomplish the difficult hurdle of transporting antimicrobials across the cell membranes of pathogens. Lessons learned from the successes and failures of siderophore-conjugate drugs evaluated during the development of novel agents using the ‘Trojan horse’ approach have been reviewed.
Examples
Antimicrobial peptides are produced by species across the tree of life, including: * bacteria (''e.g.''bacteriocin
Bacteriocins are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ec ...
, and many others)
* fungi (''e.g.'' peptaibols, plectasin, and many others)
* cnidaria (''e.g.'' hydramacin
Hydramacin-1 is a type of antimicrobial protein. It was first isolated and reproduced in 2008 from cells of the freshwater hydroid '' Hydra''. Only around 60 amino acids long, the protein is unique both in amino acid sequence and tertiary structu ...
, aurelin)
* many from insects and arthropods (''e.g.'' cecropin, attacin
Attacin is a glycine-rich protein of about 20 kDa belonging to the group of antimicrobial peptides (AMP). It is active against Gram-negative bacteria
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gr ...
, melittin
Melittin is the main component (40–60% of the dry weight) and the major pain producing substance of honeybee (''Apis mellifera'') venom. Melittin is a basic peptide consisting of 26 amino acids.
Function
The principal function of melittin a ...
, mastoparan
Mastoparan is a peptide toxin from wasp venom. It has the chemical structure Ile-Asn-Leu-Lys-Ala-Leu-Ala-Ala-Leu-Ala-Lys-Lys-Ile-Leu-NH2.
The net effect of mastoparan's mode of action depends on cell type, but seemingly always involves exocytosis ...
, drosomycin)
* amphibia, frogs (magainin
The magainins are a class of antimicrobial peptides found in the African clawed frog (''Xenopus laevis''). The peptides are cationic, generally lack a stable conformation in water but form amphipathic α-helix in membranes; their mechanism agains ...
, dermaseptin
Dermaseptins are a family of peptides isolated from skin of the frog genus ''Phyllomedusa''.
The sequence of the dermaseptins varies greatly but due to the presence of lysine residues all are cationic and most have the potential to form amphipath ...
, aurein, and others)
* birds (''e.g.'' avian defensins
Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune sig ...
)
* and mammals (''e.g.'' cathelicidin
Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the ''CAMP'' gene encodes the peptide precursor CAP-18 (18 kDa), which is proce ...
s, alpha- and beta-defensins
Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune sig ...
, regIII peptides)
Research has increased in recent years to develop artificially-engineered mimics of antimicrobial peptides such as SNAPP
Snapp is a village (nowadays more like a farm), in Jörns socken, Skellefteå Municipality, Västerbotten County, Sweden.
The village got its name in the 19th century when a geographical surveyor played with the childish rhyme '' Snipp, snapp, s ...
s, in part due to the prohibitive cost of producing naturally-derived AMPs. An example of this is the facially cationic peptide C18G, which was designed from the C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
domain of human platelet factor IV. Currently, the most widely used antimicrobial peptide is nisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium ''Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), di ...
; being the only FDA approved antimicrobial peptide, it is commonly used as an artificial preservative.
Bioinformatics
Several bioinformatic databases exist to catalogue antimicrobial peptides. The APD (the Antimicrobial Peptide Database) is the original and model database for antimicrobial peptides (https://aps.unmc.edu). Based on the APD, other databases have also been built, including ADAM (A Database of Anti-Microbial peptides), BioPD (Biologically active Peptide Database), CAMP (Collection of sequences and structures of antimicrobial peptides), DBAASP (Database of Antimicrobial Activity and Structure of Peptides), DRAMP(Data Repository of Antimicrobial Peptideand LAMP (Linking AMPs). The Antimicrobial peptide databases may be divided into two categories on the basis of the source of peptides it contains, as specific databases and general databases. These databases have various tools for antimicrobial peptides analysis and prediction. For example, the APD has a widely used calculation interface. It also provides links to many other tools. CAMP contains AMP prediction, feature calculator, BLAST search, ClustalW, VAST, PRATT, Helical wheel etc. In addition, ADAM allows users to search or browse through AMP sequence-structure relationships. Antimicrobial peptides often encompass a wide range of categories such as antifungal, antibacterial, and antituberculosis peptides. : Provides an online platform for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. is an online resource that addresses various topics such as annotations of antimicrobial peptides (AMPs) including sequence information, antimicrobial activities, post-translational modifications (PTMs), structural visualization, antimicrobial potency, target species with minimum inhibitory concentration (MIC), physicochemical properties, or AMP–protein interactions. Tools such as PeptideRanker, PeptideLocator, and AntiMPmod allow for the prediction of antimicrobial peptides while others have been developed to predict antifungal and anti-Tuberculosis activities.
See also
* Aurein *Bacteriocin
Bacteriocins are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ec ...
* Cathelicidin
Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the ''CAMP'' gene encodes the peptide precursor CAP-18 (18 kDa), which is proce ...
* Copsin
* Diptericin
Diptericin is a 9 kDa antimicrobial peptide (AMP) of flies first isolated from the blowfly '' Phormia terranova''. It is primarily active against Gram-negative bacteria, disrupting bacterial membrane integrity. The structure of this protein incl ...
* Peripheral membrane proteins
Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the periph ...
* Virtual colony count Virtual colony count (VCC) is a kinetic, 96-well microbiological assay originally developed to measure the activity of defensins. It has since been applied to other antimicrobial peptides including LL-37. It utilizes a method of enumerating bacter ...
References
External links
ADAM (A Database of Anti-Microbial peptides)
at ntou.edu.tw
AntiFP
Prediction of antifungal peptides
AntiMPmod
Prediction of antimicrobial potential of modified peptides *
AntiTbPred
Prediction of anti-tuberculosis peptides
Antimicrobial Peptide Database
at University of Nebraska Medical Center
Antimicrobial Peptide Scanner
Deep Learning based AMP prediction server
AntiTbPdb
Anti Tubercular Peptide Database
BioPD
at Peking University Health Science Center
CAMP:Collection of Anti-Microbial Peptides
at National Institute for Research in Reproductive Health (NIRRH)
DBAASP
- Database of Antimicrobial Activity and Structure of Peptides]
LAMP
at Fudan University
PeptideLocator
Prediction of functional peptides, including antimicrobial peptides, in a protein sequence
PeptideRanker
Bioactive peptide, including antimicrobial peptide, prediction
modlAMP
Python package for computational work with antimicrobial peptides, including sequence handling, -design, -prediction, descriptor calculation and plotting {{DEFAULTSORT:Antimicrobial Peptides Antimicrobial peptides, Immunology Immune system Peripheral membrane proteins Insect immunity