Ammonia production on:
[Wikipedia]
[Google]
[Amazon]

CaO + 3C <=> CaC2 + CO
: CaC2 + N2 <=> CaCN2 + C
: CaCN2 + 3H2O <=> CaCO3 + 2NH3
 ::H2S + ZnO → ZnS + H2O
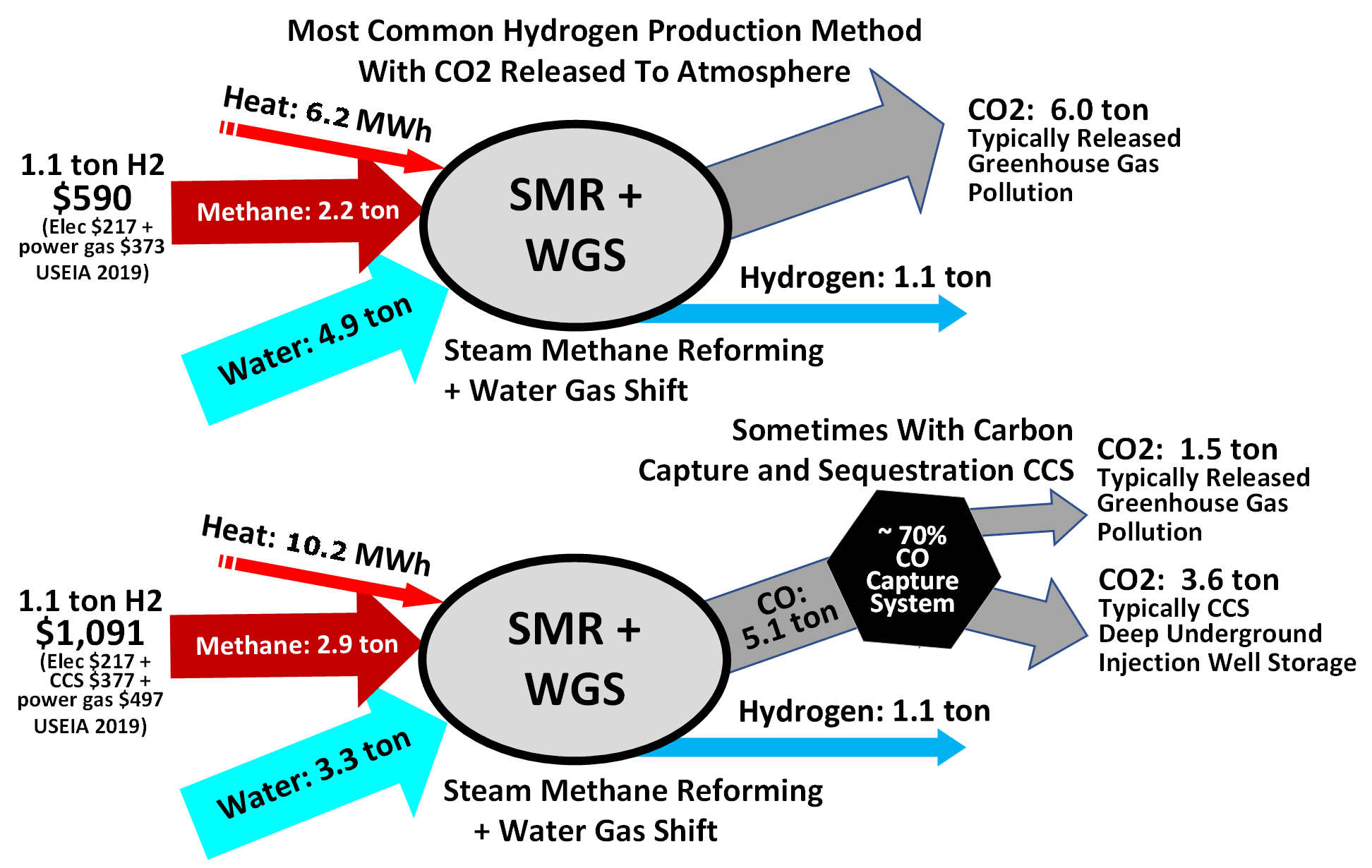
* Catalytic steam reforming of the sulfur-free feedstock is then used to form hydrogen plus
::H2S + ZnO → ZnS + H2O
* Catalytic steam reforming of the sulfur-free feedstock is then used to form hydrogen plus
 Ammonia production depends on plentiful supplies of
Ammonia production depends on plentiful supplies of 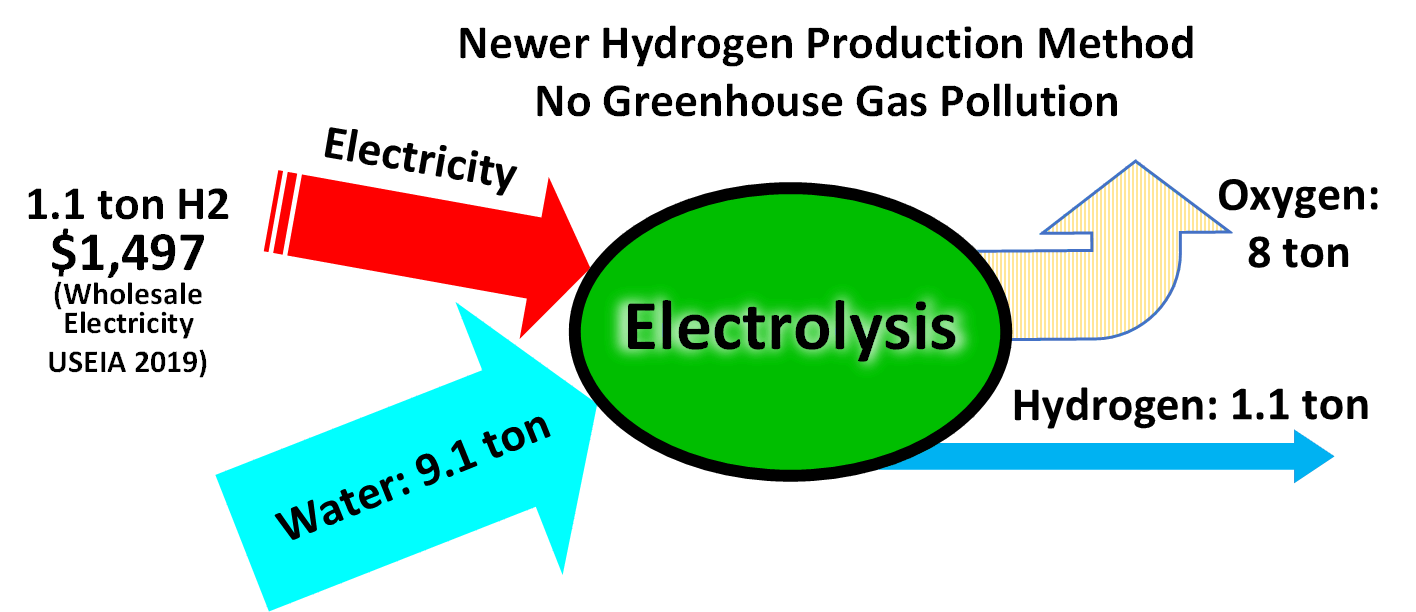 This would be straightforward in a hydrogen economy by diverting some hydrogen production from fuel to feedstock use. For example, in 2002, Iceland produced 2,000 tons of hydrogen gas by electrolysis, using excess electricity production from its
This would be straightforward in a hydrogen economy by diverting some hydrogen production from fuel to feedstock use. For example, in 2002, Iceland produced 2,000 tons of hydrogen gas by electrolysis, using excess electricity production from its
 Ammonia made from coal is a process mainly practised by China. China produced about 32.6% of the global production in 2014, while Russia, India, and the U.S. produced 8.1%, 7.6%, and 6.4%. Most of their ammonia came from coal. The basic processing in a coal-based ammonia plant consists of an air separation module for the separation of and from air, the gasifier, the sour gas shift module, the acid gas removal module, and the ammonia synthesis module. Oxygen from the air separation module is fed to the gasifier to convert coal into synthesis gas (, CO, ) and . There are many gasifier designs, but most gasifiers are based on fluidized beds that operate above atmospheric pressure and have the ability to utilize different coal feeds.
Ammonia made from coal is a process mainly practised by China. China produced about 32.6% of the global production in 2014, while Russia, India, and the U.S. produced 8.1%, 7.6%, and 6.4%. Most of their ammonia came from coal. The basic processing in a coal-based ammonia plant consists of an air separation module for the separation of and from air, the gasifier, the sour gas shift module, the acid gas removal module, and the ammonia synthesis module. Oxygen from the air separation module is fed to the gasifier to convert coal into synthesis gas (, CO, ) and . There are many gasifier designs, but most gasifiers are based on fluidized beds that operate above atmospheric pressure and have the ability to utilize different coal feeds.
Today's Hydrogen Production IndustryEnergy Use and Energy Intensity of the U.S. Chemical Industry
Report LBNL-44314,
Ammonia: The Next Step
includes a detailed process flow diagram.
Ammonia production process plant flow sheet
in brief with three controls. Ammonia Chemical processes
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
is one of the most highly produced inorganic chemicals. There are numerous large-scale ammonia plants worldwide, producing a grand total of 144 million tonnes of nitrogen (equivalent to 175 million tonnes of ammonia) in 2016. This has increased to 235 million tonnes of ammonia in 2021. China produced 31.9% of the worldwide production, followed by Russia with 8.7%, India with 7.5%, and the United States with 7.1%. 80% or more of the ammonia produced is used for fertilizing agricultural crops. Ammonia is also used for the production of plastics, fibres, explosives, nitric acid (via the Ostwald process), and intermediates for dyes and pharmaceuticals.
History
Dry distillation
Before the start ofWorld War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was List of wars and anthropogenic disasters by death toll, one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, ...
, most ammonia was obtained by the dry distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
of nitrogenous vegetable and animal products; by the reduction of nitrous acid and nitrites with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
; and also by the decomposition of ammonium salts by alkaline hydroxides or by quicklime, the salt most generally used being the chloride ( sal-ammoniac).

Frank–Caro process
Adolph Frank and Nikodem Caro found that Nitrogen could be fixed by using the same calcium carbide produced to make acetylene to form calcium-cyanamide, which could then be divided with water to form ammonia. The method was developed between 1895 and 1899. :Birkeland–Eyde process
While not strictly speaking a method of producing ammonia, nitrogen can be fixed by passing it (with oxygen) through an electric spark.Nitrides
Heating metals such as magnesium in an atmosphere of pure nitrogen produces the nitride, which when combined with water produce the metal hydroxide and ammonia.Modern ammonia production
Today, most ammonia is produced on a large scale by the Haber process with capacities of up to 3,300 tonnes per day. In this process, N2 and H2 gases are allowed to react at pressures of 200 bar. Ammonia is also processed by coal. The American Oil Co in the mid-1960s positioned a single-converter ammonia plant engineered by M. W. Kellogg at Texas City, Texas, with a capacity of 544 m.t./day. The single-train design concept was so thoroughgoing that it received the “Kirkpatrick Chemical Engineering Achievement Award” in 1967. The plant used a four-case centrifugal compressor to compress the syngas to a pressure of 152 bar, and final compression to an operating pressure of 324 bar occurred in a reciprocating compressor. Centrifugal compressors for the synthesis loop and refrigeration services were also implemented, which provided significant cost. Almost every plant built between 1964 and 1992 had large single-train designs with synthesis gas manufacturing at 25–35 bar and ammonia synthesis at 150–200 bar. Another variation by Braun (now KBR) offered slight tempering to the plain design. The Braun Purifier process plants utilized a primary or tubular reformer with a low outlet temperature and high methane leakage to reduce the size and cost of the reformer. Excess air was added to the secondary reformer to reduce the methane content of the primary reformer exit stream to 1–2%. Excess nitrogen and other impurities were erased downstream of the methanator. Because the synthesis gas was essentially free of impurities, two axial-flow ammonia converters were used to attain a high ammonia conversion. In early 2000 Uhde developed a new process which enables plant capacities of 3300 mtpd and more. The key innovation of Uhde's dual-pressure process is a single-flow synthesis loop at medium pressure in series with a conventional high-pressure synthesis loop.Modern ammonia-producing plants
A typical modern ammonia-producing plant first convertsnatural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
, liquified petroleum gas
Liquefied petroleum gas (LPG or LP gas) is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane and n-butane.
LPG is used as a fuel gas in heating appliances, cookin ...
, or petroleum naphtha
Naphtha ( or ) is a flammable liquid hydrocarbon mixture.
Mixtures labelled ''naphtha'' have been produced from natural gas condensates, petroleum distillates, and the distillation of coal tar and peat. In different industries and regions ' ...
into gaseous hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
. The method for producing hydrogen from hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s is known as steam reforming. The hydrogen is then combined with nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
to produce ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
via the Haber-Bosch process.
Starting with a natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
() feedstock, the different steps used in the process of producing hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
are the following:
* The first step in the process is to remove sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
compounds from the feedstock because sulfur deactivates the catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s used in subsequent steps. Sulfur removal requires catalytic hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
to convert sulfur compounds in the feedstocks to gaseous hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
:
::H2 + RSH → RH + H2S(gas)
* The gaseous hydrogen sulfide is then adsorbed and removed by passing it through beds of zinc oxide where it is converted to solid zinc sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various ...
:
 ::H2S + ZnO → ZnS + H2O
* Catalytic steam reforming of the sulfur-free feedstock is then used to form hydrogen plus
::H2S + ZnO → ZnS + H2O
* Catalytic steam reforming of the sulfur-free feedstock is then used to form hydrogen plus carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
:
::CH4 + H2O → CO + 3 H2
* The next step then uses catalytic shift conversion to convert the carbon monoxide to carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
and more hydrogen:
::CO + H2O → CO2 + H2
* The carbon dioxide is then removed either by absorption in aqueous ethanolamine solutions or by adsorption in pressure swing adsorbers (PSA) using proprietary solid adsorption media.
* The final step in producing the hydrogen is to use catalytic methanation to remove any small residual amounts of carbon monoxide or carbon dioxide from the hydrogen:
::CO + 3 H2 → CH4 + H2O
::CO2 + 4 H2 → CH4 + 2 H2O
To produce the desired end-product ammonia, the hydrogen is then catalytically reacted with nitrogen (derived from process air) to form anhydrous liquid ammonia. This step is known as the ammonia synthesis loop (also referred to as the Haber-Bosch process):
::3 H2 + N2 → 2 NH3
Due to the nature of the (typically multi-promoted magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With ...
) catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
used in the ammonia synthesis reaction, only very low levels of oxygen-containing (especially CO, CO2 and H2O) compounds can be tolerated in the synthesis (hydrogen and nitrogen mixture) gas. Relatively pure nitrogen can be obtained by air separation, but additional oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
removal may be required.
Because of relatively low single pass conversion rates (typically less than 20%), a large recycle stream is required. This can lead to the accumulation of inerts in the loop gas.
Pressure differentials
The steam reforming, shift conversion,carbon dioxide removal
Carbon dioxide removal (CDR), also known as negative emissions, is a process in which carbon dioxide gas () is removed from the atmosphere and sequestered for long periods of time. Similarly, greenhouse gas removal (GGR) or negative greenh ...
, and methanation steps each operate at absolute pressures of about 25 to 35 bar, and the ammonia synthesis loop operates at absolute pressures ranging from 60 to 180 bar depending upon the particular method used.
Sustainable ammonia production
 Ammonia production depends on plentiful supplies of
Ammonia production depends on plentiful supplies of energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
, predominantly natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
. Due to ammonia's critical role in intensive agriculture
Intensive agriculture, also known as intensive farming (as opposed to extensive farming), conventional, or industrial agriculture, is a type of agriculture, both of crop plants and of animals, with higher levels of input and output per unit of ag ...
and other processes, sustainable production is desirable. This is possible by using non-polluting methane pyrolysis or generating hydrogen by electrolysis of water (or steam) utilizing zero carbon electricity from renewable energy sources or nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced b ...
. In recent years thyssenkrupp Uhde Chlorine Engineers has expanded its annual production capacity for alkaline water electrolysis to 1 gigawatt of electrolyzer capacity.
 This would be straightforward in a hydrogen economy by diverting some hydrogen production from fuel to feedstock use. For example, in 2002, Iceland produced 2,000 tons of hydrogen gas by electrolysis, using excess electricity production from its
This would be straightforward in a hydrogen economy by diverting some hydrogen production from fuel to feedstock use. For example, in 2002, Iceland produced 2,000 tons of hydrogen gas by electrolysis, using excess electricity production from its hydroelectric
Hydroelectricity, or hydroelectric power, is electricity generated from hydropower (water power). Hydropower supplies one sixth of the world's electricity, almost 4500 TWh in 2020, which is more than all other renewable sources combined an ...
plants, primarily for the production of ammonia for fertilizer.
The Vemork hydroelectric plant in Norway used its surplus electricity output to generate renewable nitric acid from 1911 to 1971,
requiring 15 MWh/Ton of nitric acid. The same reaction is carried out by lightning, providing a natural source for converting atmospheric nitrogen to soluble nitrates. In practice, natural gas will remain the major source of hydrogen for ammonia production as long as it is the cheapest.
Waste water is often high in ammonia. Because discharging ammonia laden water into the environment, even in wastewater treatment plants, can cause problems, nitrification
''Nitrification'' is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria. The transformation of ...
is often necessary to remove the ammonia. This may be a potentially sustainable source of ammonia in the future because of its abundance and the need to remove it from the water anyway. Alternatively, ammonia from waste water is sent into an ammonia electrolyzer (ammonia electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
) operating with renewable energy sources (Solar PV and Wind turbine) to produce hydrogen and clean treated water. Ammonia electrolysis may require much less thermodynamic energy than water electrolysis (only 0.06 V in alkaline media).
Another option for recovering ammonia from waste water is to use the mechanics of the ammonia-water thermal absorption cycle. Using this option, ammonia can be recovered either as a liquid or as ammonium hydroxide. The advantage of the former is that it is much easier to handle and transport, whereas the latter also has a commercial value when a concentration of 30 percent ammonium hydroxide in solution is produced.
Ammonia made from coal
 Ammonia made from coal is a process mainly practised by China. China produced about 32.6% of the global production in 2014, while Russia, India, and the U.S. produced 8.1%, 7.6%, and 6.4%. Most of their ammonia came from coal. The basic processing in a coal-based ammonia plant consists of an air separation module for the separation of and from air, the gasifier, the sour gas shift module, the acid gas removal module, and the ammonia synthesis module. Oxygen from the air separation module is fed to the gasifier to convert coal into synthesis gas (, CO, ) and . There are many gasifier designs, but most gasifiers are based on fluidized beds that operate above atmospheric pressure and have the ability to utilize different coal feeds.
Ammonia made from coal is a process mainly practised by China. China produced about 32.6% of the global production in 2014, while Russia, India, and the U.S. produced 8.1%, 7.6%, and 6.4%. Most of their ammonia came from coal. The basic processing in a coal-based ammonia plant consists of an air separation module for the separation of and from air, the gasifier, the sour gas shift module, the acid gas removal module, and the ammonia synthesis module. Oxygen from the air separation module is fed to the gasifier to convert coal into synthesis gas (, CO, ) and . There are many gasifier designs, but most gasifiers are based on fluidized beds that operate above atmospheric pressure and have the ability to utilize different coal feeds.
Small-scale onsite plants
In April 2017, a company to implement a method of ammonia synthesis that could allow economic production at scales 1-2 orders of magnitude below than ordinary plants with utilizing electrochemical catalyst was established in Japan. The company, Tsubame BHB, have implemented the synthesis process into a module, and aiming to be adopted in distributed locations close to ammonia users.Byproducts and shortages due to shutdowns
One of the main industrial byproducts of ammonia production is CO2. In 2018, high oil prices resulted in an extended summer shutdown of European ammonia factories causing a commercial CO2 shortage, thus limiting production of carbonated drinks such as beer and fizzy soft drinks. This situation repeated in September 2021 due to a 250-400% increase in the wholesale price of natural gas over the course of the year.See also
*Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
* Amine gas treating
* Haber process
* Hydrogen economy
* Methane pyrolysis
References
{{ReflistExternal links
Today's Hydrogen Production Industry
Report LBNL-44314,
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL), commonly referred to as the Berkeley Lab, is a United States national laboratory that is owned by, and conducts scientific research on behalf of, the United States Department of Energy. Located in ...
(Scroll down to page 39 of 40 PDF pages for a list of the ammonia plants in the United States)Ammonia: The Next Step
includes a detailed process flow diagram.
Ammonia production process plant flow sheet
in brief with three controls. Ammonia Chemical processes