1,1,2,2-Tetrachloroethane on:
[Wikipedia]
[Google]
[Amazon]
1,1,2,2-tetrachloroethane (TeCA), also known as bonoform, cellon, or westron is a toxic, synthetic halogen rich alkane. It is colorless liquid and has a sweet odor. It is used as an industrial solvent or as a separation agent. TeCA can be inhaled, consumed or absorbed through the skin. After exposure, nausea, dizziness or even liver damage may occur.

4 studiesCoyer HA. 1944. Tetrachloroethane poisoning. Ind Med 13:230-233. Willcox WH, Spilsbury BH, Legge TM. 1915. An outbreak of toxic jaundice of a new type amongst aeroplane workers-Its clinical and toxicological aspect. Trans Med Soc London 38:129-156. Jeney E, Bartha F, Kondor L, et al. 1957. revention of industrial tetrachloroethane intoxication--Part III.Egeszsegtudomany 1:155-164. (Hungarian) on humans after TeCA exposure determined gastrointestinal distress in the participants. Two humans exposed to 2.9 ppm TeCA for 30 minutes showed symptoms of vomiting and nausea. These symptoms also caused weight loss.
A study by Horiuchi et al.Horiuchi K, Horiguchi S, Hashimoto K, et al. 1962. Studies on the industrial tetrachloroethane poisoning. Osaka City Medical J 8:29-38. showed that a monkey frequently exposed to 1.9 ppm TeCA got anorexic and developed regular diarrhea.
Workers in an artificial silk factory that had regularly inhaled TeCA, showed elevated white blood cell levels and slight anemia.Koelsch F. 1915. Industrial poisonings by celluloid varnishes in the airplane industry. Muench Medizin Wochensch 62:1567-1569.
In 1962, a study showed that of the investigated rats exposed to 9000 ppm TeCA for 29 days had decreased red blood cells and hemoglobin levels.
Autopsies on humans who died due to TeCA exposure showed that some humans developed hepatic failure from the TeCA, they showed jaundice and an enlarged liver. The liver is the most affected system with TeCA poisoning, causing for example apoptosis of the liver tissue.
After 60 ppm exposure rats show fatty liver degeneration. Another study determined the limit for acute hepatic failure to be at 102ppm for four hours, indicated by increases in hepatic ascorbic acid and serum glutamate dehydrogenase and decreases in serum triglycerides.
The vapors of TeCA can cause eye irritation, stinging, squinting and lacrimation in both humans and animals. This is due to direct contact of the skin and vapor rather than inhalation or digestion.
Inhalation of TeCA vapor can cause dizziness, headache and tremors.
Acute symptoms in rats showed in the form of 50% motor loss when exposed to 360ppm for one hour.
The National Cancer Institute performed experiments on the tumorigenicity of TeCA in rats and mice via the oral exposure route. Liver tumors were found in both species. Other studies on the tumorigenic mode of action revealed that it acts both as initiator and promoter.
History
1,1,2,2-tetrachloroethane was used in large amounts to produce other chemicals liketrichloroethylene
The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, w ...
, tetrachloroethylene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2 . It is a colorless li ...
, and 1,2,-dichloroethylene. It also found its function as a industrial solvent and was used in paint removers and pesticides.
Because of its possible carcinogen effects on humans, the production of 1,1,2,2-tetrachloroethane has decreased significantly and is no longer widely used as an end-product. It is however still generated as a byproduct and as an intermediate product during manufacturing, where low levels of the chemical have been detected in the air.
Synthesis
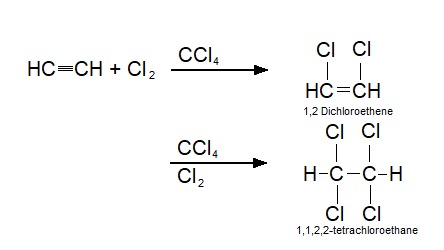
There are a few different ways to synthesise 1,1,2,2-tetrachloroethane. 1,1,2,2-tetrachloroethane can be produced by the catalytic addition of chlorine to acetylene (ethyn) which yields the highest purity. It is also produced by direct chlorination or oxychlorination utilizing ethylene as feedstock and by catalytic chlorination of ethane or chlorination of 1,2- dichloroethane. 1,1,2,2-Tetrachloroethane is always produced in closed systems to obtain the highest yield. Common side products that are created during the synthesis of 1,1,2,2-tetrachloroethane are 1,2-dichloroethane and trichloroethylene (in the presence of heat).
Reactivity
Alcohol increases the metabolism of 1,1,2,2-tetrachloroethane(TeCA) and it will intensify the effects of TeCA.Schmidt P, Binnevies S, Gohlke R, et al. 1972. [Subacute action of low concentration of chlorinated ethanes on rats with and without additional ethanol treatment. I. Biochemical and toxicometrical aspects, especially results in subacute and chronic toxicity studies with 1,1,2,2-tetrachloroethane.] Int Arch Arbeitsmed 30:283-298. (German) Humans who consume alcohol might be at an increased risk for all toxic effects from TeCA. This is also a case for several other chlorinated aliphatic hydrocarbons. An investigation showed when you combine alcohol with TeCA it increases the relative weight of the experimented rats, indicating an enlarged activity of TeCA.Metabolism
The metabolism is believed to involve cytochrome (CYP) P450. Experiments showed thatbiotransformation Biotransformation is the biochemical modification of one chemical compound or a mixture of chemical compounds. Biotransformations can be conducted with whole cells, their lysates, or purified enzymes. Increasingly, biotransformations are effected w ...
reactions increased with chronic ethanol consumption and fasting.

Toxicity
Mechanism of action
Looking at the chemical and physical properties the 1,1,2,2,-tetrachloroethane (TeCA) might be rapidly and extensively absorbed, which results in oral and inhalation exposures. In animal studies the oral take up was reported bij 70-100%Hanley TR, Quast JF, Schumann AM. 1988. The metabolism and hepatic macromolecular interactions of 1,1,2,2-tetrachloroethane (TCE) in mice and rats. Dow Chemical Company. Submitted to the U.S. Environmental Protection Agency under TSCA Section 8D. OTS0514187.Milman HA, Mitoma C, Tyson C, et al. 1984. Comparative pharmacokinetics/metabolism, carcinogenicity and mutagenicity of chlorinated ethanes and ethylenes (meeting abstract). Arbete och Halsa 29:19. and 40-97% oral uptake in human inhalation.Lehmann KB, Schmidt-Kehl L. 1936. tudy of the 13 most important chlorohydrocarbons from the standpoint of industrial hygienics.Arch Hyg 116:132-200. (German) Morgan A, Black A, Belcher DR. 1970. The excretion in breath of some aliphatic halogenated hydrocarbons following administration by inhalation. Ann Occup Hyg 13:219. TeCA is a small, volatile,lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipo ...
molecule; it appears that TeCA readily be absorbed from respiratory
The respiratory system (also respiratory apparatus, ventilatory system) is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies gre ...
and gastrointestinal
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans and ...
tracts. Absorption with passive diffusion
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to dri ...
is the most likely mechanism.
After TeCA is absorbed in the body, it is readily distributed throughout the body via passive diffusion. TeCA will most likely accumulate in lipid-rich tissues, liverYllner S. 1971. Metabolism of 1,1,2,2-tetrachloroethane-14C in the mouse. Acta Pharmacol Toxicol 29:499-512. . Urinary elimination occurs as metabolites, including formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Est ...
, glyoxalic acid, trichloroacetic acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are calle ...
and trichloroethanol
2,2,2-Trichloroethanol is the chemical compound with formula . Its molecule can be described as that of ethanol, with the three hydrogen atoms at position 2 (the methyl group) replaced by chlorine atoms. It is a clear flammable liquid at room tem ...
.Gargas ML, Andersen ME. 1989. Determining kinetic constants of chlorinated ethane metabolism in the rat from rates of exhalation. Toxicol Appl Pharmacol 99:344-353. Ikeda M, Ohtsuji H. 1972. Comparative study of the excretion of Fujiwara reaction-positive substances in urine of humans and rodents given trichloro-or tetrachloro-derivatives of ethane and ethylene. Br J Ind Med 29:99-184.
Already mentioned before passive diffusion is an important mechanism, because it is most likely the major mechanism of excretion
Excretion is a process in which metabolic waste
is eliminated from an organism. In vertebrates this is primarily carried out by the lungs, kidneys, and skin. This is in contrast with secretion, where the substance may have specific tasks after ...
.
TeCA metabolism to reactive products plays a key role in the toxicity of TeCA. Microsomal and nuclear cytochrome P450 enzymes are implicated in the metabolism with TeCA, releasing biologically active compounds as; aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
, acids
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
and free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spon ...
. Experiments of Hanley, Milman and Mitoma obtained evidence of this metabolism in rats.Milman HA, Story DL, Riccio ES, et al. 1988. Rat liver foci and in vitro assays to detect initiating and promoting effects of chlorinated ethanes and ethylenes. Ann NY Acad Sci 534:521-530. Thus, metabolic capacity for tissues high, liver, formation of active metabolites is a likely mechanism for the toxicity. DF
Mechanism for neurological
Neurology (from el, νεῦρον (neûron), "string, nerve" and the suffix -logia, "study of") is the branch of medicine dealing with the diagnosis and treatment of all categories of conditions and disease involving the brain, the spinal c ...
effects is not yet determined and therefore can not be described, TeCA might play a role. The property of the readily passive diffusion to lipid-rich tissues allows it to interfere with neural membrane function, central nervous system depression, behavioral changes and anesthesia
Anesthesia is a state of controlled, temporary loss of sensation or awareness that is induced for medical or veterinary purposes. It may include some or all of analgesia (relief from or prevention of pain), paralysis (muscle relaxation), ...
. but there are no studies of TeCA's mechanism of neuronal effects.
Mode of action of the carcinogenic effect of TeCA is not completely determined. Several studies of TeCA have reported increases in the number of hepatocytes
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, ...
in mitosis
In cell biology, mitosis () is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is maintai ...
, but the role these effects might have of TeCA on carcinogenicity is not evaluated. It suggests that TeCA may have promoting and initiating activity.
Toxicokinetics
The most common health effect was found to be on the liver following 1,1,2,2-tetrachloroethane (TeCA) exposure. The studies for this have been divided into the four different Toxicokinetic phases: Adsorption, Distribution, Metabolism and Excretion (ADME
ADME is an abbreviation in pharmacokinetics and pharmacology for "absorption, distribution, metabolism, and excretion", and describes the disposition of a pharmaceutical compound within an organism. The four criteria all influence the drug level ...
). Three exposure routes have been studied to examine the effects depending on the entry route of TeCA into the body.
# Oral exposure: The experiment for the oral exposure was done by administering oral doses of radioactively labeled TeCA by gavage in corn oil to rats and mice. Followed by measuring the radioactivity in the expired air and urine. a) Adsorption: With a measured radioactivity of 65%-73% the conclusion made was that the compound is almost completely absorbed orally. b) Distribution: Hepatic protein binding was observed by purifying the liver protein. Furthermore adverse effects were seen in liver, kidney and testes leading to the conclusion that TeCA is distributed to these tissues.National Cancer Institute (US). Division of Cancer Cause and Prevention. Bioassay of 1, 1, 2, 2-tetrachloroethane for Possible Carcinogenicity. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Cancer Institute, Division of Cancer Cause and Prevention; 1978. c) Metabolism: see experiments on Metabolism routes d) Excretion: After 72h more than 90% of the dose was excreted in metabolized or unchanged form. The largest part was excreted in breath followed by urine and the least amount of TeCA was recovered in feces. 20%-30% were retained in skin and carcass.
# Inhalation exposure: The experiment on the health effects following inhalation exposure was performed on human volunteers for adsorption and excretion studies and on animals for distribution and metabolism. A bulb containing 38C1-labeled TeCA was inserted into their mouths and the volunteers inhaled deeply, held their breath for 20 seconds, and exhaled. The excretion of the radiolabeled TeCA was measured. a) Adsorption: The results of the study showed that 97% TeCA was adsorbed in a single breath. b) Distribution: After exposure to mice and rats via inhalation adverse effects were observed in liver and kidney indicating a systemic distribution of TeCA to these tissues. c) Metabolism: Following 6 hours of inhalation exposure the level of radioactively labeled TeCA was measured at a concentration of 7.73% non metabolized in expired air. 72 hours later 1.78% was measured. d) Excretion: One hour after exposure 3% of inhaled TeCA was measured in excreted breath and 0.015% in urine.
# Dermal exposure: To measure the health effects following dermal exposure were performed 1mL of TeCA was applied to the skin of mice and guinea pigs. a) Adsorption: Within one half hour the dose was absorbed into the skin. b) Distribution: No experiments available. c) Metabolism: No experiments available d) Excretion: The half-life of TeCA in blood was shown to be approximately two hours.
Health effects
1,1,2,2-tetrachloroethane (TeCA) has a vast array of effects spread throughout the whole body. Effects have been investigated on different systems on both humans and animals, stated respectively.Lethal dose
Due to several case study reports on individuals who died after ingesting TeCA, the approximate lethal dose was possible to be established. Since the amount consumed varied this was difficult to exactly determine. One report was shown to be 4100mg/kg, the second 357mg/kg and the third 1100-9600mg/kg.Mant AK. Acute Tetrachlorethane Poisoning. British Medical Journal. 1953 Mar 21;1(4811):655. Death following the ingestion occurred within 3-20 hours.See also
*1,1,1,2-Tetrachloroethane
1,1,1,2-Tetrachloroethane is a chlorinated hydrocarbon. It is a colorless liquid with a sweet chloroform-like odor. It is used as a solvent and in the production of wood stains and varnishes.
See also
* 1,1,2,2-Tetrachloroethane 1,1,2,2-tetra ...
References