|
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic field, electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, Light, (visible) light, ultraviolet, X-rays, and gamma rays. All of these waves form part of the electromagnetic spectrum. Classical electromagnetism, Classically, electromagnetic radiation consists of electromagnetic waves, which are synchronized oscillations of electric field, electric and magnetic fields. Depending on the frequency of oscillation, different wavelengths of electromagnetic spectrum are produced. In a vacuum, electromagnetic waves travel at the speed of light, commonly denoted ''c''. In homogeneous, isotropic media, the oscillations of the two fields are perpendicular to each other and perpendicular to the direction of energy and wave propagation, forming a transverse wave. The position of an electromagnetic wave w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
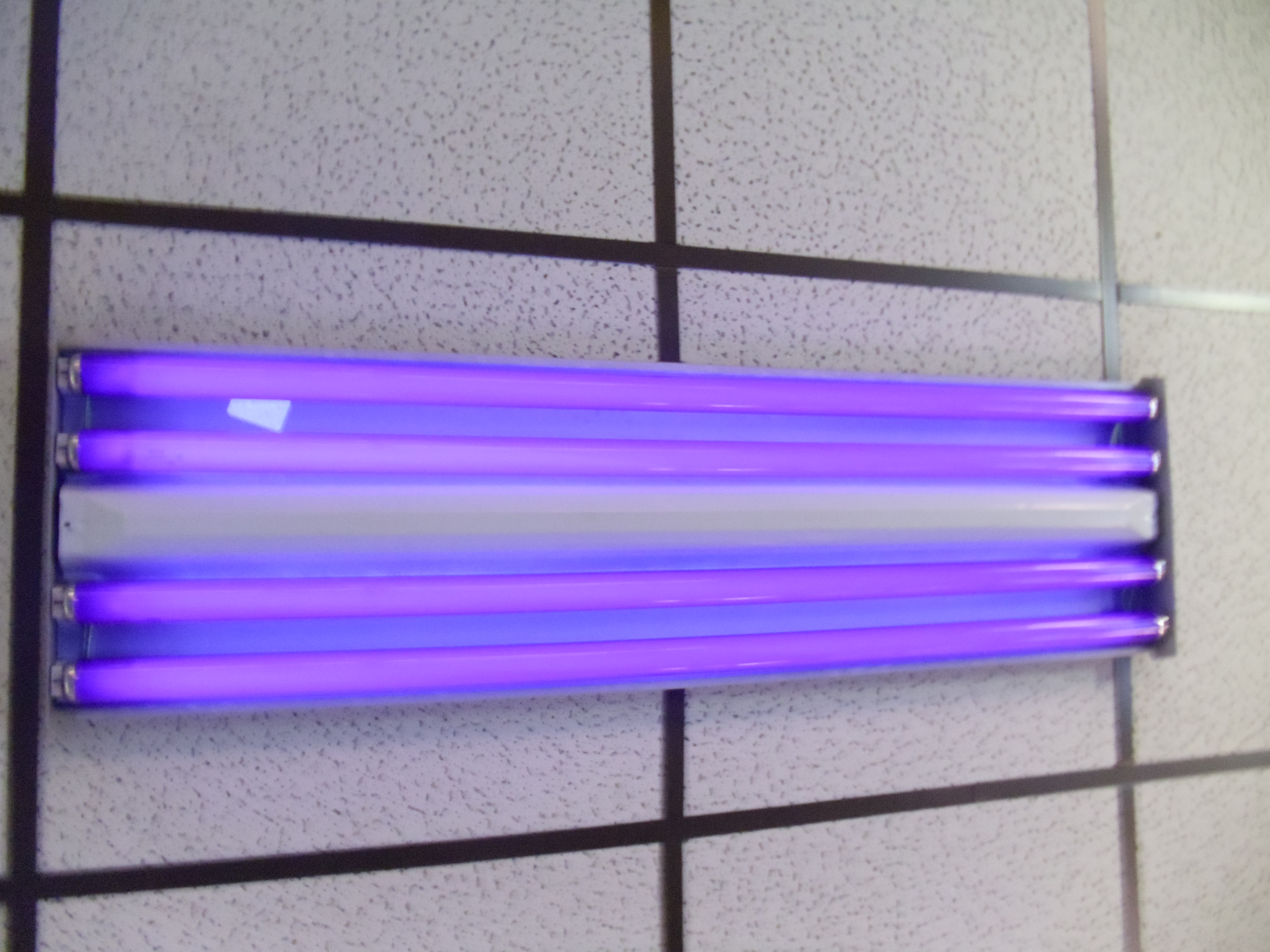
Black Light
A blacklight, also called a UV-A light, Wood's lamp, or ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and very little visible light. One type of lamp has a violet filter material, either on the bulb or in a separate glass filter in the lamp housing, which blocks most visible light and allows through UV, so the lamp has a dim violet glow when operating. Blacklight lamps which have this filter have a lighting industry designation that includes the letters "BLB". This stands for "blacklight blue". A second type of lamp produces ultraviolet but does not have the filter material, so it produces more visible light and has a blue color when operating. These tubes are made for use in "bug zapper" insect traps, and are identified by the industry designation "BL". This stands for "blacklight". Blacklight sources may be specially designed fluorescent lamps, mercury-vapor lamps, light-emitting diodes (LEDs), lasers, or incandescent lamps. In medicine, f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sun Tanning
Sun tanning or tanning is the process whereby skin color is darkened or tanned. It is most often a result of exposure to ultraviolet (UV) radiation from sunlight or from artificial sources, such as a tanning lamp found in indoor tanning beds. People who deliberately tan their skin by exposure to the sun engage in a passive recreational activity of sun bathing. Some people use chemical products which can produce a tanning effect without exposure to ultraviolet radiation, known as sunless tanning. Impact on skin health Moderate exposure to direct sunlight contributes to the production of melanin and vitamin D by the body, but excessive exposure to ultraviolet rays has negative health effects, including sunburn and increased risk of skin cancer, as well as depressed immune system function and accelerated aging of the skin. Some people tan or sunburn more easily than others. This may be the result of different skin types and natural skin color, and these may be a result of gene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sunlight
Sunlight is a portion of the electromagnetic radiation given off by the Sun, in particular infrared, visible, and ultraviolet light. On Earth, sunlight is scattered and filtered through Earth's atmosphere, and is obvious as daylight when the Sun is above the horizon. When direct solar radiation is not blocked by clouds, it is experienced as sunshine, a combination of bright light and radiant heat. When blocked by clouds or reflected off other objects, sunlight is diffused. Sources estimate a global average of between 164 watts to 340 watts per square meter over a 24-hour day; this figure is estimated by NASA to be about a quarter of Earth's average total solar irradiance. The ultraviolet radiation in sunlight has both positive and negative health effects, as it is both a requisite for vitamin D3 synthesis and a mutagen. Sunlight takes about 8.3 minutes to reach Earth from the surface of the Sun. A photon starting at the center of the Sun and changing direction eve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). The major natural source of the vitamin is synthesis of cholecalciferol in the lower layers of epidermis of the skin through a chemical reaction that is dependent on sun exposure (specifically UVB radiation). Cholecalciferol and ergocalciferol can be ingested from the diet and supplements. Only a few foods, such as the flesh of fatty fish, naturally contain significant amounts of vitamin D. In the U.S. and other countries, cow's milk and plant-derived milk substitutes are fortified with vitamin D, as are many breakfast cereals. Mushrooms exposed to ultraviolet light contribute useful amounts of vitamin D2. Dietary recommendations typically assume that all of a person's vitamin D is taken ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury-vapor Lamp
A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger soda lime or borosilicate glass bulb. The outer bulb may be clear or coated with a phosphor; in either case, the outer bulb provides thermal insulation, protection from the ultraviolet radiation the light produces, and a convenient mounting for the fused quartz arc tube. Mercury vapor lamps are more energy efficient than incandescent lamps with luminous efficacies of 35 to 55 lumens/watt. Their other advantages are a long bulb lifetime in the range of 24,000 hours and a high intensity, clear white light output. For these reasons, they are used for large area overhead lighting, such as in factories, warehouses, and sports arenas as well as for streetlights. Clear mercury lamps produce a greenish light due to mercury's combination of spectral lines. This is not flatt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Visible Light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 terahertz, between the infrared (with longer wavelengths) and the ultraviolet (with shorter wavelengths). In physics, the term "light" may refer more broadly to electromagnetic radiation of any wavelength, whether visible or not. In this sense, gamma rays, X-rays, microwaves and radio waves are also light. The primary properties of light are intensity, propagation direction, frequency or wavelength spectrum and polarization. Its speed in a vacuum, 299 792 458 metres a second (m/s), is one of the fundamental constants of nature. Like all types of electromagnetic radiation, visible light propagates by massless elementary particles called photons that represents the quanta of electromagnetic field, and can be analyzed as both waves and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sunburn
Sunburn is a form of radiation burn that affects living tissue, such as skin, that results from an overexposure to ultraviolet (UV) radiation, usually from the Sun. Common symptoms in humans and animals include: red or reddish skin that is hot to the touch or painful, general fatigue, and mild dizziness. Other symptoms include blistering, peeling skin, swelling, itching, and nausea. Excessive UV radiation is the leading cause of (primarily) non-malignant skin tumors,World Health Organization, International Agency for Research on Cance"Do sunscreens prevent skin cancer" Press release No. 132, 5 June 2000World Health Organization, International Agency for Research on Cance"Solar and ultraviolet radiation"IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 55, November 1997 and in extreme cases can be life-threatening. Sunburn is an inflammatory response in the tissue triggered by direct DNA damage by UV radiation. When the cells' DNA is overly damag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionizing Radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy ultraviolet part of the electromagnetic spectrum are ionizing radiation, whereas the lower energy ultraviolet, visible light, nearly all types of laser light, infrared, microwaves, and radio waves are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area is not sharply defined, as different molecules and atoms ionize at different energies. The energy of ionizing radiation starts between 10 electronvolts (eV) and 33 eV. Typical ionizing subatomic particles include alpha particles, beta particles, and neutrons. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tanning Lamp
Tanning lamps (sometimes called tanning bulbs in the United States or tanning tubes in Europe) are the part of a tanning bed, booth or other tanning device which produces ultraviolet light used for indoor tanning. There are hundreds of different kinds of tanning lamps most of which can be classified in two basic groups: low pressure and high pressure. Within the industry, it is common to call high-pressure units "bulbs" and low-pressure units "lamps", although there are many exceptions and not everyone follows this example. This is likely due to the size of the unit, rather than the type. Both types require an oxygen free environment inside the lamp. Fluorescent tanning lamps require an electrical ballast to limit the amount of current going through the lamp. While the resistance of an incandescent lamp filament inherently limits the current inside the lamp, tanning lamps do not and instead have negative resistance. They are plasma devices, like a neon sign, and will pass as mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skin Cancer
Skin cancers are cancers that arise from the skin. They are due to the development of abnormal cells that have the ability to invade or spread to other parts of the body. There are three main types of skin cancers: basal-cell skin cancer (BCC), squamous-cell skin cancer (SCC) and melanoma. The first two, along with a number of less common skin cancers, are known as nonmelanoma skin cancer (NMSC). Basal-cell cancer grows slowly and can damage the tissue around it but is unlikely to spread to distant areas or result in death. It often appears as a painless raised area of skin that may be shiny with small blood vessels running over it or may present as a raised area with an ulcer. Squamous-cell skin cancer is more likely to spread. It usually presents as a hard lump with a scaly top but may also form an ulcer. Melanomas are the most aggressive. Signs include a mole that has changed in size, shape, color, has irregular edges, has more than one color, is itchy or bleeds. More than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)
