|
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydrolysis of pept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsinogen
Trypsinogen () is the precursor form (or zymogen) of trypsin, a digestive enzyme. It is produced by the pancreas and found in pancreatic juice, along with amylase, lipase, and chymotrypsinogen. It is cleaved to its active form, trypsin, by enteropeptidase, which is found in the intestinal mucosa. Once activated, the trypsin can cleave more trypsinogen into trypsin, a process called autoactivation. Trypsin cleaves the peptide bond on the carboxyl side of basic amino acids such as arginine and lysine. Function Trypsinogen is the proenzyme precursor of trypsin. Trypsinogen (the inactive form) is stored in the pancreas so that it may be released when required for protein digestion. The pancreas stores the inactive form trypsinogen because the active trypsin would cause severe damage to the tissue of the pancreas. Trypsinogen is released by the pancreas into the second part of the duodenum, via the pancreatic duct, along with other digestive enzymes. Activation of trypsinogen Tryps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsinisation
Trypsinization is the process of cell dissociation using trypsin, a protease, proteolytic enzyme which breaks down proteins, to dissociate adherent cells from the vessel in which they are being cultured. When added to a cell culture, trypsin breaks down the proteins that enable the cells to adhere to the vessel. Trypsinization is often used to pass cells to a new vessel. When the trypsinization process is complete the cells will be in suspension and appear rounded. For experimental purposes, cell (biology), cells are often cultivated in containers that take the form of plastic flasks or plates. In such flasks, cells are provided with growth medium comprising the essential nutrients required for proliferation, and the cells adhere to the container and each other as they grow. This process of cell culture or tissue culture requires a method to dissociate the cells from the container and each other. Trypsin, an enzyme commonly found in the digestive tract, can be used to "digest" the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Protease
Serine proteases (or serine endopeptidases) are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like. Classification The MEROPS protease classification system counts 16 superfamilies (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evolution of the catalytic mechanism. The majority belong to the S1 family of the PA clan (superfamily) of proteases. For superfamilies, P: superfamily, containing a mixture of nucleophile class families, S: purely serine proteases. superfamily. Within each superfamily, families are designated by their catalytic nucleophile, (S: serine proteases). Substrate specificity Serine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Triad
A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An acid- base-nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence (primary structure). As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enteropeptidase
Enteropeptidase (also called enterokinase) is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen (a zymogen) into its active form trypsin, resulting in the subsequent activation of pancreas, pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment. Enteropeptidase is a serine protease () consisting of a disulfide-linked heavy-chain of 82-140 kDa that anchors enterokinase in the intestinal brush border membrane and a light-chain of 35–62 kDa that contains the catalytic subunit. Enteropeptidase is a part of the chymotrypsin-clan of serine proteases, and is structurally similar to these proteins. Historical significance Enteropeptidase was discovered by Ivan Pavlov, who was awarded the 1904 Nobel Prize in Physiology or Medicine for his studies of gastrointestinal physiology. It is the first known enzyme to activate other enzymes, and it remains a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site) for it to become an active enzyme. The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it. The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues. Although they limit the enzyme's ability, these N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit. The pancreas secretes zymogens partly to prevent the enzymes from digesting proteins in the cells in which they are synthesised. Enzymes like pepsin are created in the form of pepsinogen, an inactive zymogen. Pepsinogen is activated when chief ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholecystokinin
Cholecystokinin (CCK or CCK-PZ; from Greek ''chole'', "bile"; ''cysto'', "sac"; ''kinin'', "move"; hence, ''move the bile-sac (gallbladder)'') is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of digestive enzymes and bile from the pancreas and gallbladder, respectively, and also acts as a hunger suppressant. History Evidence that the small intestine controls the release of bile was uncovered as early as 1856, when French physiologist Claude Bernard showed that when dilute acetic acid was applied to the orifice of the bile duct, the duct released bile into the duodenum. In 1903 the French physiologist showed that this reflex was not mediated by the nervous system. In 1904 the French physiologist Charles Fleig showed that the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic period (), and the Classical period (). Ancient Greek was the language of Homer and of fifth-century Athenian historians, playwrights, and philosophers. It has contributed many words to English vocabulary and has been a standard subject of study in educational institutions of the Western world since the Renaissance. This article primarily contains information about the Epic and Classical periods of the language. From the Hellenistic period (), Ancient Greek was followed by Koine Greek, which is regarded as a separate historical stage, although its earliest form closely resembles Attic Greek and its latest form approaches Medieval Greek. There were several regional dialects of Ancient Greek, of which Attic Greek developed into Koine. Dia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerin
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
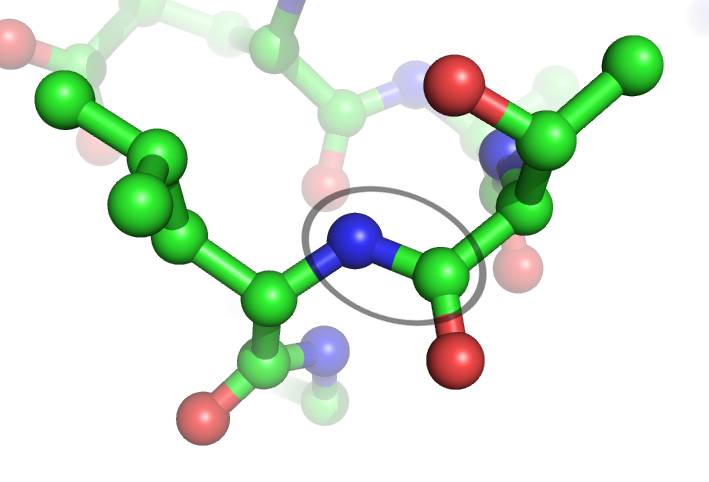
Peptide Bonds
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a ''dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. The am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duodenum
The duodenum is the first section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear, and the terms anterior intestine or proximal intestine may be used instead of duodenum. In mammals the duodenum may be the principal site for iron absorption. The duodenum precedes the jejunum and ileum and is the shortest part of the small intestine. In humans, the duodenum is a hollow jointed tube about 25–38 cm (10–15 inches) long connecting the stomach to the middle part of the small intestine. It begins with the duodenal bulb and ends at the suspensory muscle of duodenum. Duodenum can be divided into four parts: the first (superior), the second (descending), the third (horizontal) and the fourth (ascending) parts. Structure The duodenum is a C-shaped structure lying adjacent to the stomach. It is divided anatomically into four sections. The first part of the duodenum lies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |