|
Tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles are collectively known as the cytoskeleton, and one of the most ancient systems is based on filamentous polymers of the protein actin. A polymer of a second protein, tropomyosin, is an integral part of most actin filaments in animals. Tropomyosins are a large family of integral components of actin filaments that play a critical role in regulating the function of actin filaments in both muscle and nonmuscle cells. These proteins consist of rod-shaped coiled-coil hetero- or homo- dimers that lie along the α-helical groove of most actin filaments. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. Tropomyosins are often categorised into two groups, muscle tropomyosin isoforms and nonmu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropomyosin Isoform Diversity Is Generated By The Use Of Four Genes (α,β,γ,δ) And Alternative Splicing Within At Least Three Genes
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons. Tropomyosin and the actin skeleton All organisms contain organelles that provide physical integrity to their cells. These type of organelles are collectively known as the cytoskeleton, and one of the most ancient systems is based on filamentous polymers of the protein actin. A polymer of a second protein, tropomyosin, is an integral part of most actin filaments in animals. Tropomyosins are a large family of integral components of actin filaments that play a critical role in regulating the function of actin filaments in both muscle and nonmuscle cells. These proteins consist of rod-shaped coiled-coil hetero- or homo- dimers that lie along the α-helical groove of most actin filaments. Interaction occurs along the length of the actin filament, with dimers aligning in a head-to-tail fashion. Tropomyosins are often categorised into two groups, muscle tropomyosin isoforms and nonmu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TPM2
β-Tropomyosin, also known as tropomyosin beta chain is a protein that in humans is encoded by the ''TPM2'' gene. β-tropomyosin is striated muscle-specific coiled coil dimer that functions to stabilize actin filaments and regulate muscle contraction. Structure β-tropomyosin is roughly 32 kDa in molecular weight (284 amino acids), but multiple splice variants exist. Tropomysin is a flexible protein homodimer or heterodimer composed of two alpha-helical chains, which adopt a bent coiled coil conformation to wrap around the seven actin molecules in a functional unit of muscle. It is polymerized end to end along the two grooves of actin filaments and provides stability to the filaments. Tropomyosin dimers are composed of varying combinations of tropomyosin isoforms; human striated muscles express protein from the ''TPM1'' (α-tropoomyosin), ''TPM2'' (β-tropomyosin) and ''TPM3'' (γ-tropomyosin) genes, with α-tropomyosin being the predominant isoform in striated muscle. Fast sk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TPM1
Tropomyosin alpha-1 chain is a protein that in humans is encoded by the ''TPM1'' gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells. Structure Tm is a 32.7 kDa protein composed of 284 amino acids. Tm is a flexible protein homodimer or heterodimer composed of two alpha-helical chains, which adopt a bent coiled coil conformation to wrap around the seven actin molecules in a functional unit of muscle. It is polymerized end to end along the two grooves of actin filaments and provides stability to the filaments. Human striated muscles express protein from the ''TPM1'' (α-Tm), ''TPM2'' (β-Tm) and ''TPM3'' (γ-Tm) genes, with α-Tm being the predominant isoform in striated muscle. In human cardiac muscle the ratio of α-Tm to β-Tm is roughly 5:1. Function Tm functions in association with the troponin complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropomyosin 3
Tropomyosin alpha-3 chain is a protein that in humans is encoded by the ''TPM3'' gene In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b .... This gene encodes a member of the tropomyosin family of actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells. Tropomyosins are dimers of coiled-coil proteins that polymerize end-to-end along the major groove in most actin filaments. They provide stability to the filaments and regulate access of other actin-binding proteins. In muscle cells, they regulate muscle contraction by controlling the binding of myosin heads to the actin filament. Mutations in this gene result in autosomal dominant nemaline myopathy, and oncogenes formed by chromosomal translocations involving this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the mobility and contraction of cells during cell division. Actin participates in many important cellular processes, including muscle contraction, cell motility, cell division and cytokinesis, vesicle and organelle movement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
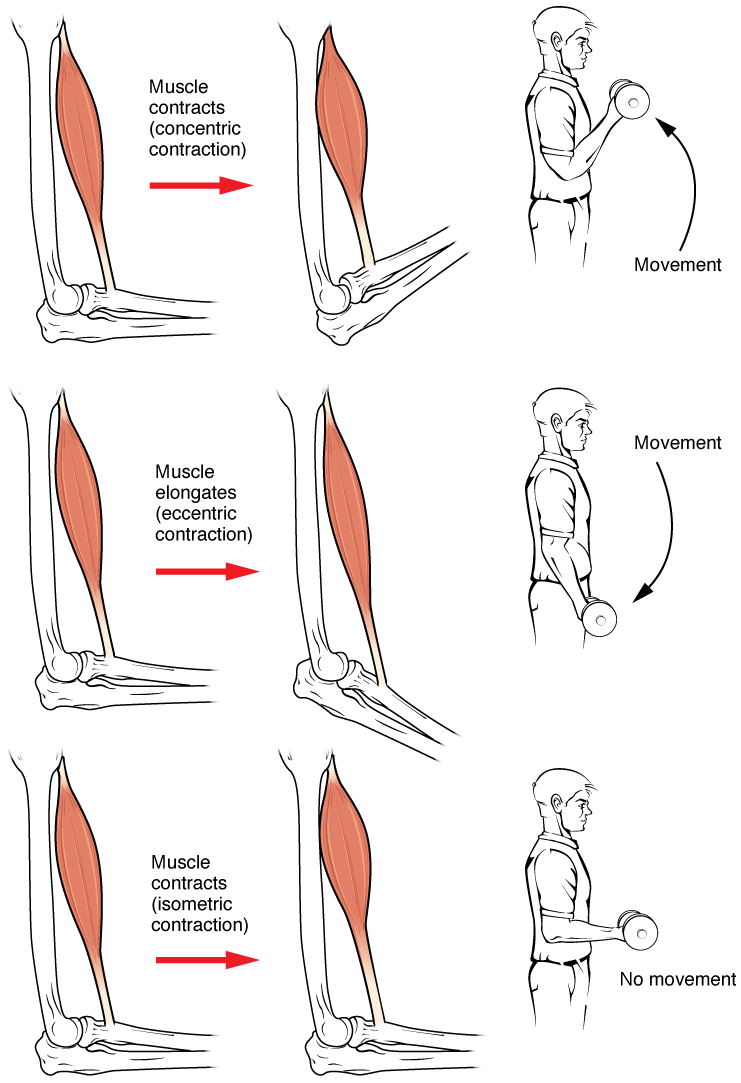
Muscle Contraction
Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state. For the contractions to happen, the muscle cells must rely on the interaction of two types of filaments which are the thin and thick filaments. Thin filaments are two strands of actin coiled around each, and thick filaments consist of mostly elongated proteins called myosin. Together, these two filaments form myofibrils which are important organelles in the skeletal muscle system. Muscle contraction can also be described based on two variables: length and tension. A muscle contraction is described as isometric if the muscle tension changes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TPM4
Tropomyosin alpha-4 chain is a protein that in humans is encoded by the ''TPM4'' gene In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b .... References Further reading * * * * * * * * * * * * * {{gene-19-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
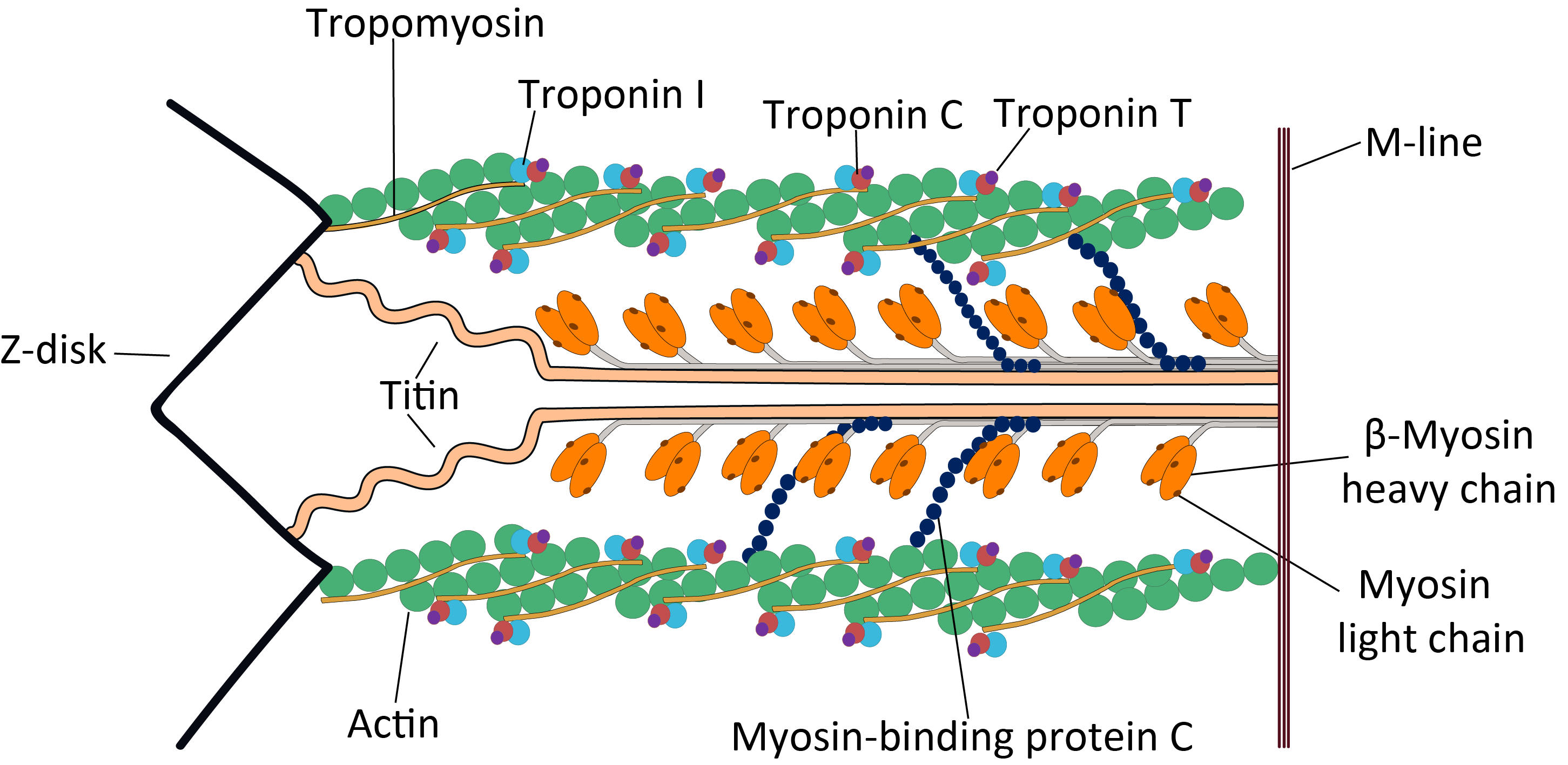
Sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called muscle fibers or myofibers) which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long, fibrous tail and a globular head, which binds to actin. The myosin head also binds to ATP, which is the source of energy for muscle movement. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiac Sarcomere Structure
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest. In humans, other mammals, and birds, the heart is divided into four chambers: upper left and right atria and lower left and right ventricles. Commonly the right atrium and ventricle are referred together as the right heart and their left counterparts as the left heart. Fish, in contrast, have two chambers, an atrium and a ventricle, while most reptiles have three chambers. In a healthy heart blood flows one way through the heart due to heart valves, which prevent backflow. The heart is enclosed in a protective sac, the pericardium, which also contains a small amount of fluid. The wal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coiled Coil
A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope. (Dimers and trimers are the most common types.) Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where Crick worked. Pauling and Crick met and spoke about various topics; at one point, Crick asked whether Pauling had considered "coiled coils" (Crick came up with the term), to which Pauling said he had. Upon returning to the United States, Pauling resumed research on the topic. He con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helical
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Ast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |