|
Traxoprodil
Traxoprodil (developmental code name CP-101606) is a drug developed by Pfizer which acts as an NMDA antagonist, selective for the NR2B subunit. It has neuroprotective, analgesic, and anti-Parkinsonian effects in animal studies. Traxoprodil has been researched in humans as a potential treatment to lessen the damage to the brain after stroke, but results from clinical trials showed only modest benefit. The drug was found to cause EKG abnormalities (QT prolongation) and its clinical development was stopped. More recent animal studies have suggested traxoprodil may exhibit rapid-acting antidepressant effects similar to those of ketamine, although there is some evidence for similar psychoactive side effects and abuse potential at higher doses, which might limit clinical acceptance of traxoprodil for this application. Traxoprodil showed ketamine-like rapidly-acting antidepressant effects in a small clinical trial of 30 patients with depression who were non-responders to 6&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rislenemdaz
Rislenemdaz (developmental code names CERC-301, MK-0657) is an orally-active, selective NMDA receptor subunit 2B (NR2B) antagonist which is under development by Cerecor in the United States as an adjunctive therapy for treatment-resistant depression (TRD). In November 2013, phase II clinical trials were initiated, and in the same month, rislenemdaz received Fast Track Designation from the Food and Drug Administration for TRD. A pilot study was published in 2012, and a phase II trial was completed in 2014, but was deemed insufficient. A second attempt at a phase II trial in 2016 also found that the drug failed to demonstrate efficacy against depression. Pharmacology Rislenemdaz is a small-molecule antagonist of the NMDA receptor. The NMDA receptor is composed of several subunits, but rislenemdaz is specific for the GluN2B subunits which are only seen in the spinal cord and forebrain. Rislenemdaz binds specifically to the GluN2B subunit in order to prevent endogenous glutamat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NR2B
Glutamate MDAreceptor subunit epsilon-2, also known as ''N''-methyl D-aspartate receptor subtype 2B (NMDAR2B or NR2B), is a protein that in humans is encoded by the ''GRIN2B'' gene. NMDA receptors ''N''-methyl-D-aspartate (NMDA) receptors are a class of ionotropic glutamate receptors. The NMDA receptor channel has been shown to be involved in long-term potentiation, an activity-dependent increase in the efficiency of synaptic transmission thought to underlie certain kinds of memory and learning. NMDA receptor channels are heterotetramers composed of two molecules of the key receptor subunit NMDAR1 (GRIN1) and two drawn from one or more of the four NMDAR2 subunits: NMDAR2A (GRIN2A), NMDAR2B (GRIN2B), NMDAR2C (GRIN2C), and NMDAR2D (GRIN2D). The NR2 subunit acts as the agonist binding site for glutamate, one of the predominant excitatory neurotransmitter receptors in the mammalian brain. Function NR2B has been associated with age- and visual-experience-dependent plasticity in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfizer
Pfizer Inc. ( ) is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer (1824–1906) and his cousin Charles F. Erhart (1821–1891). Pfizer develops and produces medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company has several blockbuster drugs or products that each generate more than billion in annual revenues. In 2020, 52% of the company's revenues came from the United States, 6% came from each of China and Japan, and 36% came from other countries. Pfizer was a component of the Dow Jones Industrial Average stock market index from 2004 to August 2020. The company ranks 64th on the Fortune 500 and 49th on the Forbes Global 2000. History 1849–1950: Early history Pfizer was founded in 1849 by Charles Pfizer and Charles F. Erhart, two cousins who ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures. In general, placebos can affect how patients perceive their condition and encourage the body's chemical processes for relieving pain and a few other symptoms, but have no impact on the disease itself. Improvements that patients experience after being treated with a placebo can also be due to unrelated factors, such as regression to the mean (a statistical effect where an unusually high or low measurement is likely to be followed by a less extreme one). The use of placebos in clinical medicine raises ethical concerns, especially if they are disguised as an active treatment, as this introduces dishonesty into the doctor–patient relationship and bypasses informed consent. While it was once assumed that this deception was necessary for placebos to have any effect, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
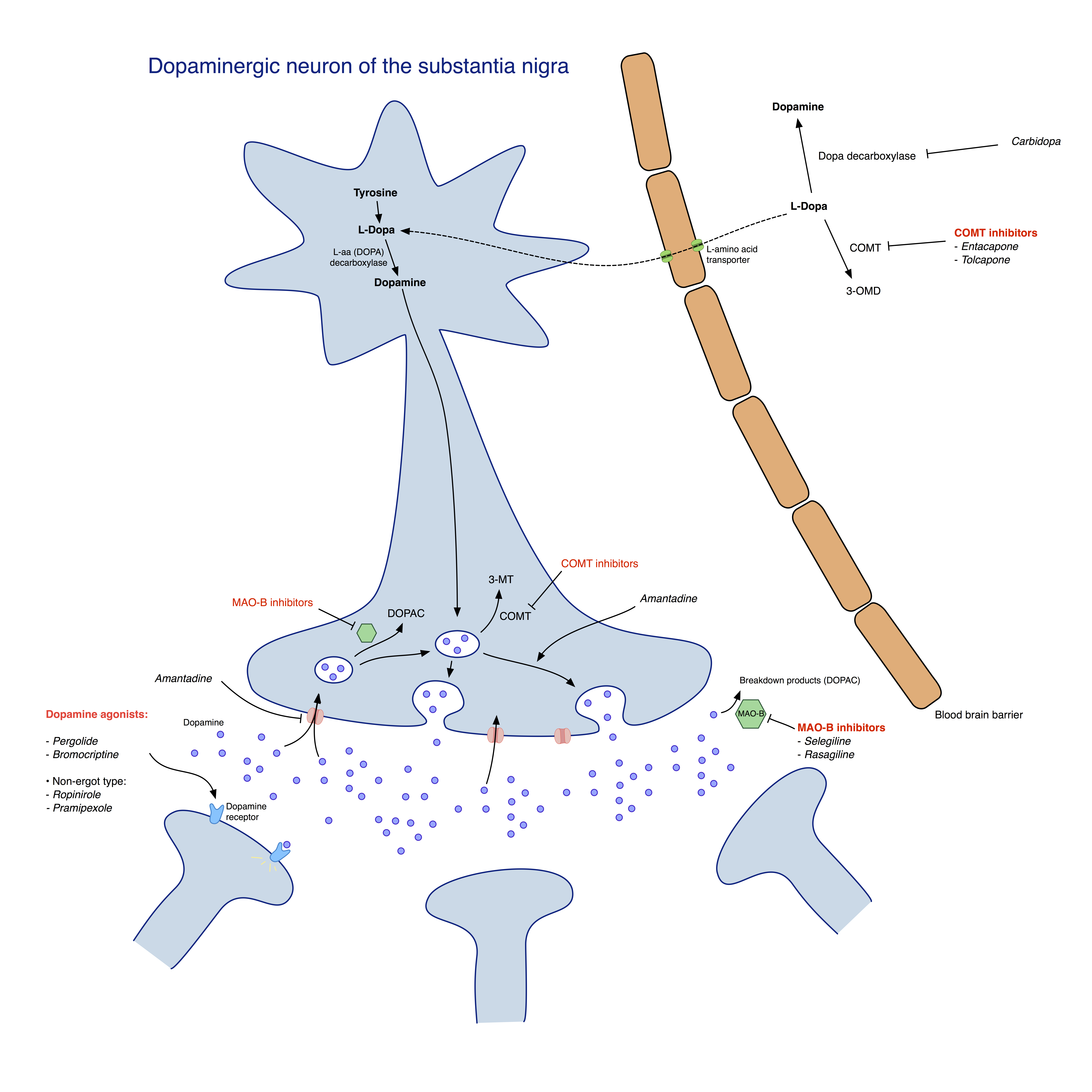
Antiparkinsonian Agents
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms. While many medications treat Parkinson's, none actually reverses the effects of the disease. Furthermore, the gold-standard treatment varies with the disease state. People with Parkinson's, therefore, often must take a variety of medications to manage the disease's symptoms. Several medications currently in development seek to better address motor fluctuations and nonmotor symptoms of PD. However, none is yet on the market with specific approval to treat Parkinson's. Medication The main families of drugs useful for treating motor symptoms are levodopa, dopamine agonists, and MAO-B inhibitors. The most commonly used treatm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antidepressants
Antidepressants are a class of medication used to treat major depressive disorder, anxiety disorders, chronic pain conditions, and to help manage addictions. Common side-effects of antidepressants include dry mouth, weight gain, dizziness, headaches, sexual dysfunction, and emotional blunting. There is a slight increased risk of suicidal thinking and behavior when taken by children, adolescents, and young adults. Discontinuation syndrome may occur after stopping any antidepressant which resembles recurrent depression. Some research regarding the effectiveness of antidepressants for depression in adults has found benefits, whilst other research has not. Evidence of benefit in children and adolescents is unclear. The twenty-one most commonly prescribed antidepressant medications are more effective than placebo for the short-term (acute) treatments of adults with major depressive disorder. There is debate in the medical community about how much of the observed effects of antidepr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesics
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Investigational Antidepressants
This is a list of investigational antidepressants, or antidepressants that are currently under development for clinical use in the treatment of mood disorders but are not yet approved. ''Chemical/generic names are listed first, with developmental code names, synonyms, and brand names in parentheses.'' All drugs listed are specifically under development for major depressive disorder (MDD) and/or treatment-resistant depression (TRD) unless noted otherwise. Other forms of depression may include bipolar depression and postpartum depression. Glutamatergics NMDA receptor modulators * 4-Chlorokynurenine (AV-101) – NMDA receptor glycine site antagonist * Apimostinel (GATE-202, NRX-1074) – NMDA receptor modulator * Arketamine (PCN-101, HR-071603) – unknown mechanism of action, indirect AMPA receptor activator * Esketamine (Esketamine DPI, Falkieri, PG061) – non-competitive NMDA receptor antagonist – approved for TRD, specifically under development for bipolar depression an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
QTc Prolongation
QT prolongation is a measure of delayed ventricular repolarisation, which means the heart muscle takes longer than normal to recharge between beats. It is an electrical disturbance which can be seen on an electrocardiogram (ECG). Excessive QT prolongation can trigger tachycardias such as torsades de pointes (TdP). QT prolongation is an established side effect of antiarrhythmics, but can also be caused by a wide range of non-cardiac medicines, including antibiotics, antihistamines, opioids, and complementary medicines. On an ECG, the QT interval represents the summation of action potentials in cardiac muscle cells, which can be caused by an increase in inward current through sodium or calcium channels, or a decrease in outward current through potassium channels. By binding to and inhibiting the “rapid” delayed rectifier potassium current protein, certain drugs are able to decrease the outward flow of potassium ions and extend the length of phase 3 myocardial repolarization, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketamine
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a recreational drug. It is one of the safest anesthetics, as, in contrast with opiates, ether, and propofol, it suppresses neither respiration nor heart rate. Ketamine is also simple to administer and highly tolerable compared to drugs with similar effects which are flammable, irritating, or even explosive. Ketamine is a novel compound, derived from PCP, created in pursuit of a safer anesthetic with similar characteristics. Ketamine is also used for acute pain management. At anesthetic doses, ketamine induces a state of "dissociative anesthesia", a trance-like state providing pain relief, sedation, and amnesia. The distinguishing features of ketamine anesthesia are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation. At lower, sub-anesthetic doses, ketamine is a promising agent fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paroxetine
Paroxetine, sold under the brand names Paxil and Seroxat among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive-compulsive disorder, panic disorder, social anxiety disorder, posttraumatic stress disorder, generalized anxiety disorder and premenstrual dysphoric disorder. It has also been used in the treatment of premature ejaculation and hot flashes due to menopause. It is taken by mouth. Common side effects include drowsiness, dry mouth, loss of appetite, sweating, trouble sleeping, and sexual dysfunction. Serious side effects may include suicidal thoughts in those under the age of 25, serotonin syndrome, and mania. While the rate of side effects appears similar compared to other SSRIs and SNRIs, antidepressant discontinuation syndromes may occur more often. Use in pregnancy is not recommended, while use during breastfeeding is relatively safe. It is believed to work by blocking th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |