|
Technetium
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only. Many of technetium's properties had been predicted by Dmitri Mendeleev before it was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name '' ekamanganese'' (''Em''). In 1937, technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world. Technetium-99m is used as a radioactive tracer and can be detected in the body by medical equipment ( gamma cameras). It is well suited to the role, because it emits readily detectable gamma rays with a photon energy of 140 keV (these 8.8 pm photons are about the same wavelength as emitted by conventional X-ray diagnostic equipment) and its half-life for gamma emission is 6.0058 hours (meaning 93.7% of it decays to 99Tc in 24 hours). The relatively "short" physical half-life of the isotope and its biological half-life of 1 day (in terms of human activity and metabolism) allows for scanning procedures which collect data rapidly but keep total patient radiation exposure low. The same characteristics ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 7 Element
Group 7, numbered by IUPAC nomenclature, is a group of elements in the periodic table. They are manganese (Mn), technetium (Tc), rhenium (Re), and bohrium (Bh). All known elements of group 7 are transition metals. Like other groups, the members of this family show patterns in their electron configurations, especially the outermost shells resulting in trends in chemical behavior. Chemistry Like other groups, the members of this family show patterns in its electron configuration, especially the outermost shells: Bohrium has not been isolated in pure form. History Manganese was discovered much earlier than the other group 7 elements owing to its much larger abundance in nature. While Johan Gottlieb Gahn is credited with the isolation of manganese in 1774, Ignatius Kaim reported his production of manganese in his dissertation in 1771. Group 7 contains the two naturally occurring transition metals discovered last: technetium and rhenium. Rhenium was discovered when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Technetium
Technetium (43Tc) is one of the two elements with that have no stable isotopes; the other such element is promethium. – Elements marked with a * have no stable isotope: 43, 61, and 83 and up. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission (there are an estimated grams of 99Tc per gram of pitchblende) or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc (half-life of 4.21 million years), 98Tc (half-life: 4.2 million years), and 99Tc (half-life: 211,100 years). Thirty-three other radioisotopes have been characterized with atomic masses ranging from 85Tc to 120Tc. Most of these have half-lives that are less than an hour; the exceptions are 93Tc (half-life: 2.75 hours), 94Tc (half-life: 4.883 hours), 95Tc (half-life: 20 hours), and 96Tc (half-life: 4.28 days). Technetium also has numerous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium-97
Technetium (43Tc) is one of the two elements with that have no stable isotopes; the other such element is promethium. – Elements marked with a * have no stable isotope: 43, 61, and 83 and up. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission (there are an estimated grams of 99Tc per gram of pitchblende) or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc ( half-life of 4.21 million years), 98Tc (half-life: 4.2 million years), and 99Tc (half-life: 211,100 years). Thirty-three other radioisotopes have been characterized with atomic masses ranging from 85Tc to 120Tc. Most of these have half-lives that are less than an hour; the exceptions are 93Tc (half-life: 2.75 hours), 94Tc (half-life: 4.883 hours), 95Tc (half-life: 20 hours), and 96Tc (half-life: 4.28 days). Technetium also has num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were created artificially are strictly speaking not ''synthetic'' because they were later found in nature in trace quantities: 43Tc, 61Pm, 85At, 93Np, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium-99
Technetium-99 (99Tc) is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting beta particles, but no gamma rays. It is the most significant long-lived fission product of uranium fission, producing the largest fraction of the total long-lived radiation emissions of nuclear waste. Technetium-99 has a fission product yield of 6.0507% for thermal neutron fission of uranium-235. The metastable technetium-99m (99mTc) is a short-lived (half-life about 6 hours) nuclear isomer used in nuclear medicine, produced from molybdenum-99. It decays by isomeric transition to technetium-99, a desirable characteristic, since the very long half-life and type of decay of technetium-99 imposes little further radiation burden on the body. Radiation The weak beta emission is stopped by the walls of laboratory glassware. Soft X-rays are emitted when the beta particles are stopped, but as long as the body is kept more than 30 cm away these should pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
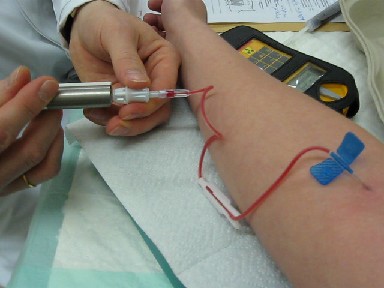
Nuclear Medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine. Diagnostic medical imaging Diagnostic In nuclear medicine imaging, radiopharmaceuticals are taken internally, for example, through inhalation, intravenously or orally. Then, external detectors ( gamma cameras) capture and form images from the radiation emitted by the radiopharmaceuticals. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state. The word ''nuclide'' was coined by Truman Paul Kohman, Truman P. Kohman in 1947. Kohman defined ''nuclide'' as a "species of atom characterized by the constitution of its nucleus" containing a certain number of neutrons and protons. The term thus originally focused on the nucleus. Nuclides vs isotopes A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, while the isotope concept (grouping all atoms of each element) emphasizes chemical over nuclear. The neutron number has large effects on nuclear properties, but its kinetic isotope effect, effect on chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by as well as , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mendeleev's Predicted Elements
Dmitri Mendeleev published a periodic table of the chemical elements in 1869 based on properties that appeared with some regularity as he laid out the elements from lightest to heaviest. When Mendeleev proposed his periodic table, he noted gaps in the table and predicted that then-unknown elements existed with properties appropriate to fill those gaps. He named them eka-boron, eka-aluminium, eka-silicon, and eka-manganese, with respective atomic masses of 44, 68, 72, and 100. Prefixes To give provisional names to his predicted elements, Mendeleev used the prefixes '' eka''- , '' dvi''- or '' dwi-'', and '' tri''-, from the Sanskrit names of digits 1, 2, and 3, depending upon whether the predicted element was one, two, or three places down from the known element of the same group in his table. For example, germanium was called eka-silicon until its discovery in 1886, and rhenium was called dvi-manganese before its discovery in 1926. The ''eka-'' prefix was used by other theor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a free metal on Earth; it is found only in various oxidation states in minerals. The free element, a silvery metal with a grey cast, has the sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum compounds have low solubility ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



%2C_black_and_white.png)
