|
Solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a wikt:saturated#Chemistry, saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscibility, miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Rate Of Solution
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Solubility Equilibrium
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent ''solubility product'' which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios. Definitions A solubility equilibrium exists when a chemical compound in the solid state is in chemical equilibrium with a solution containing the compound. This type of equilibrium is an example of dynamic equilibrium in that some individual molecules migrate between the solid and solution phases such that the rates of dissolution and precipitation are equal to one another. When equilibrium is established and the solid has not al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
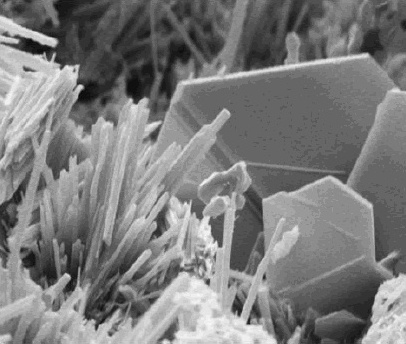
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide. Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Solubility Calcium hydroxide is moderately soluble in water, as seen for many dihydroxides. Its solubility increases from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. Its solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Solute
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, tempe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for Chemical polarity#Polarity of molecules, polar molecules, and the most common solvent used by living things; all the ions and proteins in a Cell (biology), cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for Organic compound, organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents (D-limonene, citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Supersaturation
In physical chemistry, supersaturation occurs with a solution (chemistry), solution when the concentration of a solute exceeds the concentration specified by the value of solubility at Solubility equilibrium, equilibrium. Most commonly the term is applied to a solution of a solid in a liquid, but it can also be applied to liquids and gases dissolved in a liquid. A supersaturated solution is in a metastable state; it may return to equilibrium by separation process, separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent. History Early studies of the phenomenon were conducted with sodium sulfate, also known as Glauber's Salt because, unusually, the solubility of this salt in water may decrease with increasing temperature. Early studies have been summarised by Tomlinson. It was shown that the crystallization of a supersaturated solution does not simply come from its agitation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Miscibility
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically equivalent to the common term " mixable"). The term is most often applied to liquids but also applies to solids and gases. An example in liquids is the miscibility of water and ethanol as they mix in all proportions. By contrast, substances are said to be immiscible if the mixture does not form a solution for certain proportions. For one example, oil is not soluble in water, so these two solvents are immiscible. As another example, butanone (methyl ethyl ketone) is immiscible in water: it is soluble in water up to about 275 grams per liter, but will separate into two phases beyond that. Organic compounds In organic compounds, the weight percent of hydrocarbon chain often determines the compound's miscibility with water. For examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Geology
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth sciences, including hydrology. It is integrated with Earth system science and planetary science. Geology describes the structure of the Earth on and beneath its surface and the processes that have shaped that structure. Geologists study the mineralogical composition of rocks in order to get insight into their history of formation. Geology determines the relative ages of rocks found at a given location; geochemistry (a branch of geology) determines their absolute ages. By combining various petrological, crystallographic, and paleontological tools, geologists are able to chronicle the geological history of the Earth as a whole. One aspect is to demonstrate the age of the Earth. Geology provides evidence for plate tectonics, the ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Precipitation Diagram Multilang
A chemical substance is a unique form of matter with constant chemical composition and characteristic Chemical property, properties. Chemical substances may take the form of a single chemical element, element or chemical compounds. If two or more chemical substances can be combined without Chemical reaction, reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be Chemical purity, chemically pure. Chemical substances can exist in several different physical State of matter, states or Phase (matter), phases (e.g. solids, liquids, gases, or plasma (physics), plasma) without changing their chemical composition. Substances Phase transition, transition between these phases of matter in response to changes in temperature or pressure. Some chemical substances can be combined or converted into new substances by means of chemical reactions. Chemicals that do not possess this ability ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |