|
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) below 0°C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form immedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation Of The Equilibrium Phase (red) From A Metastable Phase (white) In The Ising Model
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) below 0°C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form immedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Classical Nucleation Theory
Classical nucleation theory (CNT) is the most common theoretical model used to quantitatively study the kinetics of nucleation.H. R. Pruppacher and J. D. Klett, ''Microphysics of Clouds and Precipitation'', Kluwer (1997)P.G. Debenedetti, ''Metastable Liquids: Concepts and Principles'', Princeton University Press (1997) Nucleation is the first step in the spontaneous formation of a new thermodynamic phase or a new structure, starting from a state of metastability. The kinetics of formation of the new phase is frequently dominated by nucleation, such that the time to nucleate determines how long it will take for the new phase to appear. The time to nucleate can vary by orders of magnitude, from negligible to exceedingly large, far beyond reach of experimental timescales. One of the key achievements of classical nucleation theory is to explain and quantify this immense variation. Description The central result of classical nucleation theory is a prediction for the ''rate of nucleati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Classical Nucleation Theory
Classical nucleation theory (CNT) is the most common theoretical model used to quantitatively study the kinetics of nucleation.H. R. Pruppacher and J. D. Klett, ''Microphysics of Clouds and Precipitation'', Kluwer (1997)P.G. Debenedetti, ''Metastable Liquids: Concepts and Principles'', Princeton University Press (1997) Nucleation is the first step in the spontaneous formation of a new thermodynamic phase or a new structure, starting from a state of metastability. The kinetics of formation of the new phase is frequently dominated by nucleation, such that the time to nucleate determines how long it will take for the new phase to appear. The time to nucleate can vary by orders of magnitude, from negligible to exceedingly large, far beyond reach of experimental timescales. One of the key achievements of classical nucleation theory is to explain and quantify this immense variation. Description The central result of classical nucleation theory is a prediction for the ''rate of nucleati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubule Nucleation
In cell biology, microtubule nucleation is the event that initiates '' de novo'' formation of microtubules (MTs). These filaments of the cytoskeleton typically form through polymerization of α- and β-tubulin dimers, the basic building blocks of the microtubule, which initially interact to nucleate a seed from which the filament elongates. Microtubule nucleation occurs spontaneously ''in vitro'', with solutions of purified tubulin giving rise to full-length polymers. The tubulin dimers that make up the polymers have an intrinsic capacity to self-aggregate and assemble into cylindrical tubes, provided there is an adequate supply of GTP. The kinetics barriers of such a process, however, mean that the rate at which microtubules spontaneously nucleate is relatively low. Role of γ-tubulin and the γ-tubulin ring complex (γ-TuRC) ''In vivo'', cells get around this kinetic barrier by using various proteins to aid microtubule nucleation. The primary pathway by which microtubule nucleati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercooled
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−55 °F). Droplets of supercooled water often exist in stratus and cumulus clouds. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes. Animals utilize supercooling to survive in extreme temperatures. There are many mechanisms that aid in maintaining a liquid state, such as the production of antifreeze proteins, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice. The winter flounder is on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−55 °F). Droplets of supercooled water often exist in stratus and cumulus clouds. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes. Animals utilize supercooling to survive in extreme temperatures. There are many mechanisms that aid in maintaining a liquid state, such as the production of antifreeze proteins, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice. The winter flounder is on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, organell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing
Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid or the liquid content of a substance, usually due to cooling. For most substances, the melting and freezing points are the same temperature; however, certain substances possess differing solid-liquid transition temperatures. For example, agar displays a hysteresis in its melting point and freezing point. It melts at 85 °C (185 °F) and solidifies from 32 °C to 40 °C (89.6 °F to 104 °F). Crystallization Most liquids freeze by crystallization, formation of crystalline solid from the uniform liquid. This is a first-order thermodynamic phase transition, which means that as long as solid and liquid coexist, the temperature of the whole system remains very nearly equal to the melting point due to the slow re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid
Amyloids are aggregates of proteins characterised by a Fibril, fibrillar morphology of 7–13 Nanometer, nm in diameter, a beta sheet (β-sheet) Secondary structure of proteins, secondary structure (known as cross-β) and ability to be Staining, stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal Protein structure, structure and physiology, physiological functions (Protein misfolding, misfolding) and form fibrous deposits in amyloid plaques around cells which can disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are Genetic disorder, familial. Others are only Genetic disorder, fam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinodal Decomposition
Spinodal decomposition is a mechanism by which a single thermodynamic phase spontaneously separates into two phases (without nucleation). Decomposition occurs when there is no thermodynamic barrier to phase separation. As a result, phase separation via decomposition does not require the nucleation events resulting from thermodynamic fluctuations, which normally trigger phase separation. Spinodal decomposition is observed when mixtures of metals or polymers separate into two co-existing phases, each rich in one species and poor in the other. When the two phases emerge in approximately equal proportion (each occupying about the same volume or area), characteristic intertwined structures are formed that gradually coarsen (see animation). The dynamics of spinodal decomposition is commonly modeled using the Cahn–Hilliard equation. Spinodal decomposition is fundamentally different from nucleation and growth. When there is a nucleation barrier to the formation of a second phase, time i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Impurity
In chemistry and materials science, impurities are chemical substances inside a confined amount of liquid, gas, or solid, which differ from the chemical composition of the material or compound. Firstly, a pure chemical should appear thermodynamically in at least one chemical phase and can also be characterized by its one-component-phase diagram. Secondly, practically speaking, a pure chemical should prove to be homogeneous (i.e., will show no change of properties after undergoing a wide variety of consecutive analytical chemical procedures). The perfect pure chemical will pass all attempts and tests of further separation and purification. Thirdly, and here we focus on the common chemical definition, it should not contain any trace of any other kind of chemical species. In reality, there are no absolutely 100% pure chemical compounds, as there is always some minute contamination. Indeed, as detection limits in analytical chemistry decrease, the number of impurities detected tend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
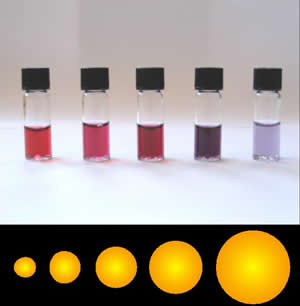
Gold Nanoparticle
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either wine-red coloured (for spherical particles less than 100 nm) or blue/purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass colloidal gold was used in the 4th-century Lycurgus Cup, which changes color depending on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)