|
NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance (NMR) Basic Principles
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla (unit), tesla, the frequency is similar to VHF and UHF Television channel frequencies, television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. The principle of NMR usually involves three sequential steps: # The alignment (polarization) of the magnetic nuclear spins in an applied, constant magnetic field B0. # The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy. Some atomic nuclei possess a magnetic moment ( nuclear spin), which gives rise to different energy levels and resonance frequencies in a magnetic field. The total magnetic field experienced by a nucleus includes local magnetic fields induced by currents of electrons in the molecular orbitals (note that electrons have a magnetic moment themselves). The electron distribution of the same type of nucleus (e.g. ) usually varies according to the local geometry (binding partners, bond lengths, angles between bonds, and so on), and with it the local magnetic field at each nucleus. This is reflected in the spin energy le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from CT and PET scans. MRI is a medical application of nuclear magnetic resonance (NMR) which can also be used for imaging in other NMR applications, such as NMR spectroscopy. MRI is widely used in hospitals and clinics for medical diagnosis, staging and follow-up of disease. Compared to CT, MRI provides better contrast in images of soft-tissues, e.g. in the brain or abdomen. However, it may be perceived as less comfortable by patients, due to the usually longer and louder measurements with the subject in a long, confining tube, though "Open" MRI designs mostly relieve this. Additionally, implants and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
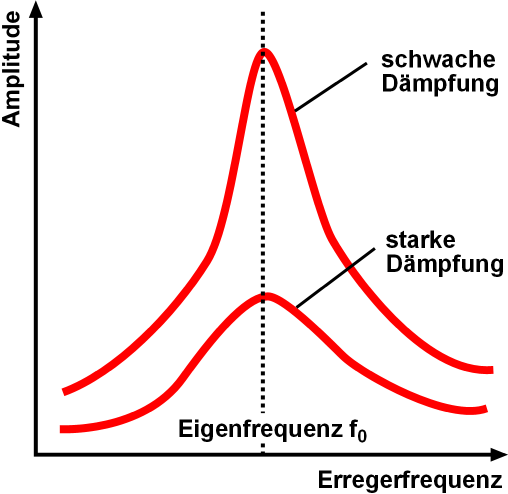
Resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies. Frequencies at which the response amplitude is a relative maximum are also known as resonant frequencies or resonance frequencies of the system. Small periodic forces that are near a resonant frequency of the system have the ability to produce large amplitude oscillations in the system due to the storage of vibrational energy. Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, orbital resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance (ESR) and reso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knight Shift
The Knight shift is a shift in the nuclear magnetic resonance (NMR) frequency of a paramagnetic substance first published in 1949 by the UC Berkeley physicist Walter D. Knight. For an ensemble of ''N'' spins in a magnetic induction field \vec, the nuclear Hamiltonian for the Knight shift is expressed in Cartesian form by: =-\sum\limits_^, where for the ''i''th spin _ is the gyromagnetic ratio, is a vector of the Cartesian nuclear angular momentum operators, the =\left( \begin & & \\ & & \\ & & \\ \end \right) matrix is a second-rank tensor similar to the chemical shift shielding tensor. The Knight shift refers to the relative shift ''K'' in NMR frequency for atoms in a metal (e.g. sodium) compared with the same atoms in a nonmetallic environment (e.g. sodium chloride). The observed shift reflects the local magnetic field produced at the sodium nucleus by the magnetization of the conduction electrons. The average local field in sodium augments the applied r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Larmor Precession
In physics, Larmor precession (named after Joseph Larmor) is the precession of the magnetic moment of an object about an external magnetic field. The phenomenon is conceptually similar to the precession of a tilted classical gyroscope in an external torque-exerting gravitational field. Objects with a magnetic moment also have angular momentum and effective internal electric current proportional to their angular momentum; these include electrons, protons, other fermions, many atomic and nuclear physics, nuclear systems, as well as classical macroscopic systems. The external magnetic field exerts a torque on the magnetic moment, :\vec = \vec\times\vec = \gamma\vec\times\vec, where \vec is the torque, \vec is the magnetic dipole moment, \vec is the angular momentum vector, \vec is the external magnetic field, \times symbolizes the cross product, and \gamma is the gyromagnetic ratio which gives the proportionality constant between the magnetic moment and the angular momentum. The an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first", and this name was given to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Moment
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is mainly a magnetic dipole moment; the quadrupole moment does cause some small shifts in the hyperfine structure as well. All nuclei that have nonzero spin also possess a nonzero magnetic moment and vice versa, although the connection between the two quantities is not straightforward or easy to calculate. The nuclear magnetic moment varies from isotope to isotope of an element. For a nucleus of which the numbers of protons and of neutrons are ''both'' even in its ground state (i.e. lowest energy state), the nuclear spin and magnetic moment are both always zero. In cases with odd numbers of either or both protons and neutrons, the nucleus often has nonzero spin and magnetic moment. The nuclear magnetic moment is not sum of nucleon magnetic moments, this property being assigned to the tensorial character of the nuclear force, such as in the case of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particles give rise to a magnetic field, which acts on other currents and magnetic moments. Magnetism is one aspect of the combined phenomena of electromagnetism. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetized to become permanent magnets, producing magnetic fields themselves. Demagnetizing a magnet is also possible. Only a few substances are ferromagnetic; the most common ones are iron, cobalt, and nickel and their alloys. The rare-earth metals neodymium and samarium are less common examples. The prefix ' refers to iron because permanent magnetism was first observed in lodestone, a form of natural iron ore called magnetite, Fe3O4. All substances exhibit some type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |