|
Lomustine
Lomustine ( INN; abbreviated as CCNU; original brand name CeeNU, now marketed as Gleostine) is an alkylating nitrosourea compound used in chemotherapy. It is closely related to semustine and is in the same family as streptozotocin. It is a highly lipid-soluble drug, thus it crosses the blood–brain barrier. This property makes it ideal for treating brain tumors, which is its primary use, although it is also used to treat Hodgkin lymphoma as a second-line option. It has also been used in veterinary practice as a treatment for cancers in cats and dogs. Lomustine is a bifunctional alkylating agent, alkylates both DNA and RNA, has the ability to created interstrand cross-links (ICLs) in DNA. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. Lomustine is cell-cycle nonspecific. Medical uses Chemotherapy in human medicine Lomustine is an alkylating chemotherapy drug that is indicated by the FDA for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lomustine Synthesis
Lomustine (INN; abbreviated as CCNU; original brand name CeeNU, now marketed as Gleostine) is an alkylating nitrosourea compound used in chemotherapy. It is closely related to semustine and is in the same family as streptozotocin. It is a highly lipid-soluble drug, thus it crosses the blood–brain barrier. This property makes it ideal for treating brain tumors, which is its primary use, although it is also used to treat Hodgkin lymphoma as a second-line option. It has also been used in veterinary practice as a treatment for cancers in cats and dogs. Lomustine is a bifunctional alkylating agent, alkylates both DNA and RNA, has the ability to created interstrand cross-links (ICLs) in DNA. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. Lomustine is cell-cycle nonspecific. Medical uses Chemotherapy in human medicine Lomustine is an alkylating chemotherapy drug that is indicated by the FDA for the tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Semustine
Semustine (1-(2-chloroethyl)-3-(''trans''-4-methylcyclohexyl)-1-nitrosourea, MeCCNU) is an alkylating nitrosourea compound used in chemotherapy treatment of various types of tumours. Due to its lipophilic property, semustine can cross the blood-brain barrier for the chemotherapy of brain tumours, where it interferes with DNA replication in the rapidly-dividing tumour cells. Semustine, just as lomustine, is administered orally. Evidence has been found that treatment with semustine can cause acute leukaemia as a delayed effect in very rare cases. Structure and reactivity Semustine (Me-CCNU) is an organochlorine compound that is urea in which the two hydrogens on one of the amino groups are replaced by nitroso and 2-chloroetyl groups and one hydrogen from the other amino group is replaced by a 4-methylcyclohexcyl group. Semustine is also known as a 4-methyl derivative of lomustine. Synthesis The synthesis of semustine originates from a systematic synthesis scheme revolving a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glioblastoma Foundation
The Glioblastoma Foundation (GF) is a United States non-profit charitable organization established in June 2016 in Durham, North Carolina. Operating as a 501(c)(3) organization, the Glioblastoma Foundation focuses on research, providing support, and promoting awareness for glioblastoma, a highly aggressive form of brain cancer. In February of 2023, Morgan Myles, country music artist and finalist on NBC's Season 22 of The Voice, was announced as the Glioblastoma Foundation's celebrity ambassador. Myles has actively participated in digital campaigns organized by the foundation, aimed at raising awareness about glioblastoma and garnering funding for the cause. Research Funding The Glioblastoma Foundation funds research on discovering new and more effective treatments for glioblastoma patients. Grants are allocated to clinicians and scientists affiliated with institutions including Johns Hopkins Hospital, MD Anderson Cancer Center, and Columbia University. The Glioblastoma Found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy regimen, regimen. Chemotherapy may be given with a cure, curative intent (which almost always involves combinations of drugs), or it may aim only to prolong life or to Palliative care, reduce symptoms (Palliative care, palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called ''oncology#Specialties, medical oncology''. The term ''chemotherapy'' now means the non-specific use of intracellular poisons to inhibit mitosis (cell division) or to induce DNA damage (naturally occurring), DNA damage (so that DNA repair can augment chemotherapy). This meaning excludes the more-selective agents that block extracellular signals (signal transduction) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrosoureas
Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea. Examples Examples include: * Arabinopyranosyl-N-methyl-N-nitrosourea, Arabinopyranosyl-''N''-methyl-''N''-nitrosourea (Aranose) * Carmustine (BCNU, BiCNU) * Chlorozotocin * Ethylnitrosourea (ENU) * Fotemustine * Lomustine (CCNU) * Nimustine * N-Nitroso-N-methylurea, ''N''-Nitroso-''N''-methylurea (NMU) * Ranimustine (MCNU) * Semustine * Streptozocin (Streptozotocin) Nitrosourea compounds are DNA alkylating antineoplastic agent, alkylating agents and are often used in chemotherapy. They are lipophile, lipophilic and thus can cross the blood–brain barrier, making them useful in the treatment of brain tumors such as glioblastoma multiforme. File:Aranose (Haworth).svg, Arabinopyranosyl-N-methyl-N-nitrosourea, Arabinopyranosyl-''N''-methyl-''N''-nitrosourea File:Carmustine.svg, Carmustine File:Chlorozotocin (Haworth).svg, Chlorozotocin File:ENU.svg, Ethylnitrosourea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrosourea Compounds
Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea. Examples Examples include: * Arabinopyranosyl-''N''-methyl-''N''-nitrosourea (Aranose) * Carmustine (BCNU, BiCNU) * Chlorozotocin * Ethylnitrosourea (ENU) * Fotemustine * Lomustine (CCNU) * Nimustine * ''N''-Nitroso-''N''-methylurea (NMU) * Ranimustine (MCNU) * Semustine * Streptozocin (Streptozotocin) Nitrosourea compounds are DNA alkylating agents and are often used in chemotherapy. They are lipophilic and thus can cross the blood–brain barrier, making them useful in the treatment of brain tumors such as glioblastoma multiforme. File:Aranose (Haworth).svg, Arabinopyranosyl-''N''-methyl-''N''-nitrosourea File:Carmustine.svg, Carmustine File:Chlorozotocin (Haworth).svg, Chlorozotocin File:ENU.svg, Ethylnitrosourea File:Fotemustine.svg, Fotemustine File:Lomustine.svg, Lomustine File:N-Nitroso-N-methylurea.svg, ''N''-Nitroso-''N''-methylurea File:Nimustine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
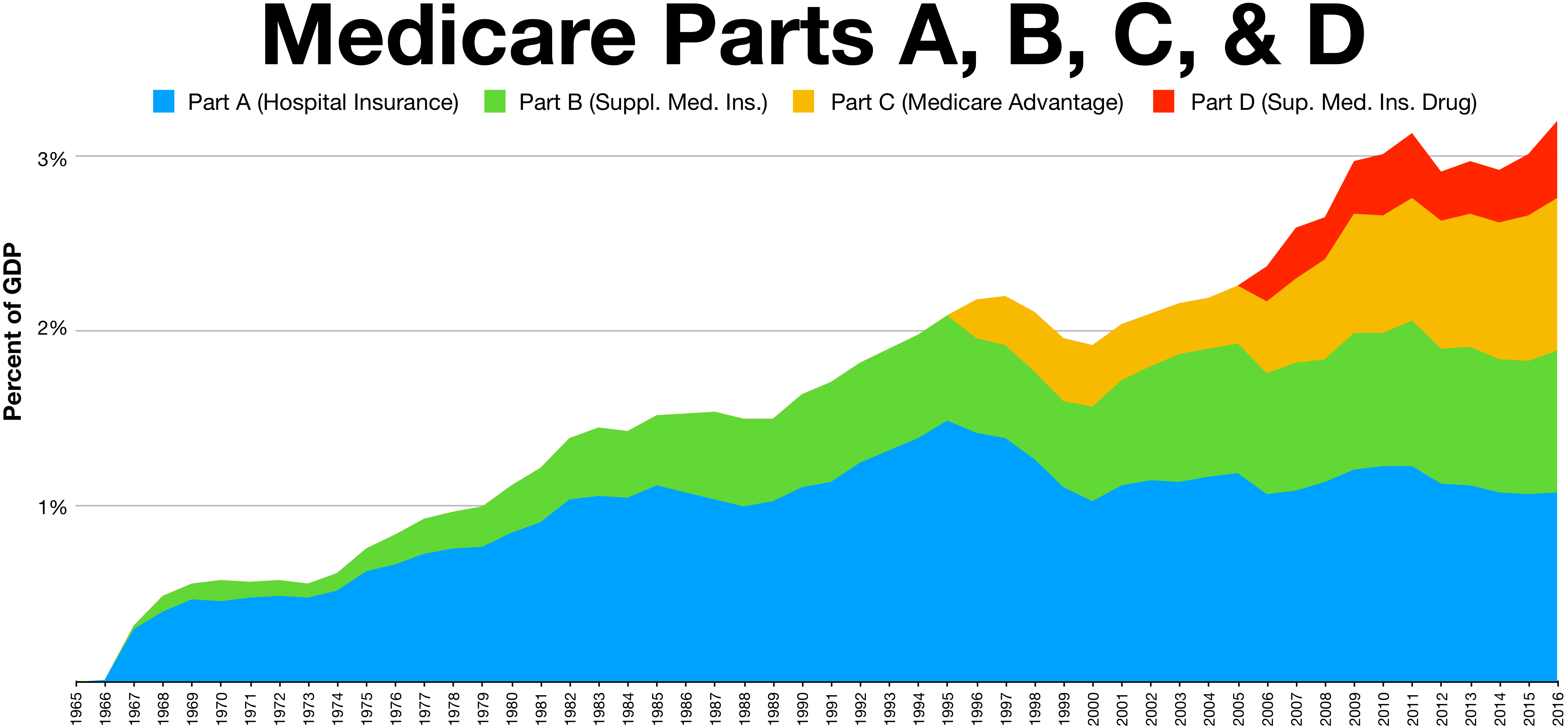
Medicare (United States)
Medicare is a federal health insurance program in the United States for people age 65 or older and younger people with disabilities, including those with End Stage Renal Disease Program, end stage renal disease and amyotrophic lateral sclerosis (ALS or Lou Gehrig's disease). It started in 1965 under the Social Security Administration and is now administered by the Centers for Medicare and Medicaid Services (CMS). Medicare is divided into four parts: A, B, C and D. Part A covers hospital, skilled nursing, and hospice services. Part B covers outpatient services. Part D covers self-administered prescription drugs. Part C is an alternative that allows patients to choose private plans with different benefit structures that provide the same services as Parts A and B, usually with additional benefits. In 2022, Medicare provided health insurance for 65.0 million individuals—more than 57 million people aged 65 and older and about 8 million younger people. According to annual Medicare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cancer Treatments
Cancer treatments are a wide range of treatments available for the many different types of cancer, with each cancer type needing its own specific treatment. Treatments can include surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy including small-molecule drugs or monoclonal antibodies, and PARP inhibitors such as olaparib. Other therapies include hyperthermia, immunotherapy, photodynamic therapy, and stem-cell therapy. Most commonly cancer treatment involves a series of separate therapies such as chemotherapy before surgery. Angiogenesis inhibitors are sometimes used to enhance the effects of immunotherapies. The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient. Biomarker testing can help to determine the type of cancer, and indicate the best therapy. A number of experimental cancer treatments are continuously under development. In 2023 it was estimated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrosamines
Nitrosamines (or more formally ''N''-nitrosamines) are organic compounds produced by industrial processes. The chemical structure is , where R is usually an alkyl group. Nitrosamines have a nitroso group () that are "probable human carcinogens", bonded to a deprotonated amine. Most nitrosamines are carcinogenic in animals. A 2006 systematic review supports a "positive association between nitrite and nitrosamine intake and gastric cancer, between meat and processed meat intake and gastric cancer and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and gastric cancer, but is not conclusive". Chemistry The organic chemistry of nitrosamines is well developed with regard to their syntheses, their structures, and their reactions. They usually are produced by the reaction of nitrous acid () and secondary amines, although other nitrosyl sources (e.g. , , Alkyl nitrite, RONO) have the same effect: : The nitrous acid usually arises from protonation of a nit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
IARC Group 2A Carcinogens
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as ''sufficient evidence'' of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is ''inadequate evidence'' of carcinogenicity in humans along with ''sufficient evidence'' of carcinogenicity in experimental animals and ''strong evidence'' that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of ''limited evidence'' of carcinogenicity in humans. This list is focusing on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alkylating Antineoplastic Agents
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA. Since cancer cells, in general, proliferate faster and with less error-correcting than healthy cells, cancer cells are more sensitive to DNA damage—such as being alkylated. Alkylating agents are used to treat several cancers. However, they are also toxic to normal cells (cytotoxic), particularly Labile cell, cells that divide frequently, such as those in the gastrointestinal tract, bone marrow, testicles and ovaries, which can cause loss of fertility. Most of the alkylating agents are also carcinogenic. History Before their use in chemotherapy, alkylating agents were better known for their use as sulfur mustard, ("mustard gas") and related chemical weapons in World War I. The nitrogen mustards were the first alkylating agents used medically, as well as the first modern cancer chemotherapies. Goodman, Gilman, and others began studying nitrogen mustar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Continuous Flow Manufacturing
Continuity or continuous may refer to: Mathematics * Continuity (mathematics), the opposing concept to discreteness; common examples include ** Continuous probability distribution or random variable in probability and statistics ** Continuous game, a generalization of games used in game theory ** Law of continuity, a heuristic principle of Gottfried Leibniz * Continuous function, in particular: ** Continuity (topology), a generalization to functions between topological spaces ** Scott continuity, for functions between posets ** Continuity (set theory), for functions between ordinals ** Continuity (category theory), for functors ** Graph continuity, for payoff functions in game theory * Continuity theorem may refer to one of two results: ** Lévy's continuity theorem, on random variables ** Kolmogorov continuity theorem, on stochastic processes * In geometry: ** Parametric continuity, for parametrised curves ** Geometric continuity, a concept primarily applied to the conic sec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |