|
Hydroxyapatite
Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. Hydroxyapatite is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride, chloride or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. Up to 50% by volume and 70% by weight of human bone is a modified form of hydroxyapatite, known as bone mineral. Carbonated calcium-deficient hydroxyapatite is the main mineral of which dental enamel and dentin are composed. Hydroxyapatite crystals are also found in pathological calcifications such as those found in breast tumor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
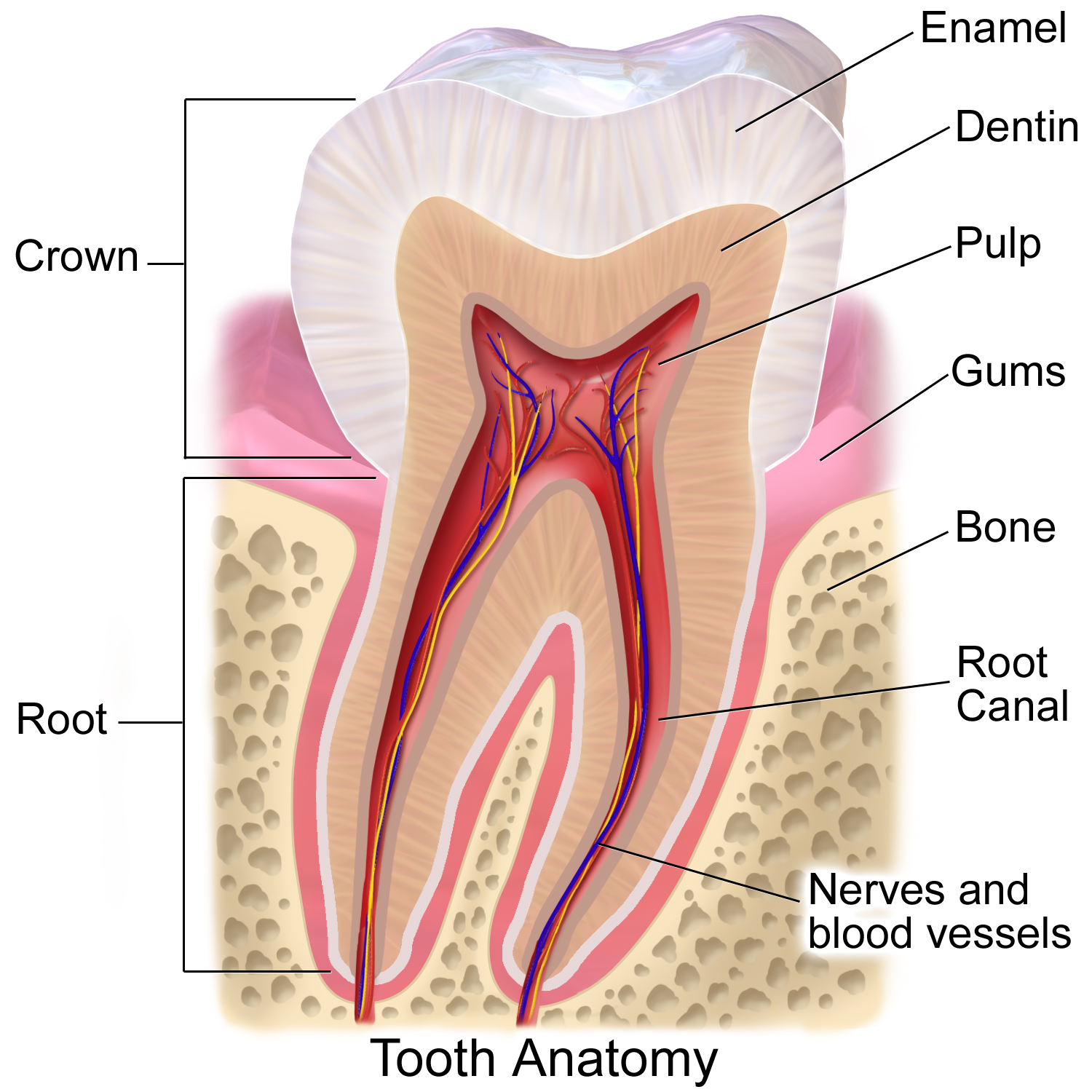
Dental Enamel
Tooth enamel is one of the four major tissues that make up the tooth in humans and many other animals, including some species of fish. It makes up the normally visible part of the tooth, covering the crown. The other major tissues are dentin, cementum, and dental pulp. It is a very hard, white to off-white, highly mineralised substance that acts as a barrier to protect the tooth but can become susceptible to degradation, especially by acids from food and drink. Calcium hardens the tooth enamel. In rare circumstances enamel fails to form, leaving the underlying dentin exposed on the surface. Features Enamel is the hardest substance in the human body and contains the highest percentage of minerals (at 96%),Ross ''et al.'', p. 485 with water and organic material composing the rest.Ten Cate's Oral Histology, Nancy, Elsevier, pp. 70–94 The primary mineral is hydroxyapatite, which is a crystalline calcium phosphate. Enamel is formed on the tooth while the tooth develops within t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorapatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, occurr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apatite
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ions, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10( PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2. The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means ''to deceive''. Geology Apatite is very common as an accessory mineral in igneous and metamorphic rocks, where it is the most common phosphate mineral. However, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in food ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corpora Arenacea
Corpora arenacea (''singular'': corpus arenaceum, also called brain sand or acervuli or psammoma bodies or pineal concretions) are calcified structures in the pineal gland and other areas of the brain such as the choroid plexus. Older organisms have numerous corpora arenacea, whose function, if any, is unknown. Concentrations of "brain sand" increase with age, so the pineal gland becomes increasingly visible on X-rays over time, usually by the third or fourth decade. They are sometimes used as anatomical landmarks in radiological examinations. Chemical analysis shows that they are composed of calcium phosphate (later characterized as hydroxyapatite), calcium carbonate, magnesium phosphate, and ammonium phosphate. Recently, calcite Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dentin
Dentin () (American English) or dentine ( or ) (British English) ( la, substantia eburnea) is a calcified tissue of the body and, along with enamel, cementum, and pulp, is one of the four major components of teeth. It is usually covered by enamel on the crown and cementum on the root and surrounds the entire pulp. By volume, 45% of dentin consists of the mineral hydroxyapatite, 33% is organic material, and 22% is water. Yellow in appearance, it greatly affects the color of a tooth due to the translucency of enamel. Dentin, which is less mineralized and less brittle than enamel, is necessary for the support of enamel. Dentin rates approximately 3 on the Mohs scale of mineral hardness. There are two main characteristics which distinguish dentin from enamel: firstly, dentin forms throughout life; secondly, dentin is sensitive and can become hypersensitive to changes in temperature due to the sensory function of odontoblasts, especially when enamel recedes and dentin channels bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bone Mineral
Bone mineral (also called inorganic bone phase, bone salt, or bone apatite) is the inorganic component of bone tissue. It gives bones their compressive strength. Bone mineral is formed predominantly from carbonated hydroxyapatite with lower crystallinity. Bone mineral is formed from globular and plate structures distributed among the collagen fibrils of bone and forming yet a larger structure. The bone salt and collagen fibers together constitute the extracellular matrix of bone tissue. Often the plural form "bone salts" is used; it reflects the notion of various salts that, on the level of molecular metabolism, can go into the formation of the hydroxyapatite. Bone mineral is dynamic in living animals; it is continually being resorbed and built anew in the bone remodeling process. In fact, the bones function as a bank or storehouse in which calcium can be continually withdrawn for use or deposited for storage, as dictated by homeostasis, which maintains the concentration of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Bone
The human skeleton is the internal framework of the human body. It is composed of around 270 bones at birth – this total decreases to around 206 bones by adulthood after some bones get fused together. The bone mass in the skeleton makes up about 14% of the total body weight (ca. 10–11 kg for an average person) and reaches maximum density around age 21. The human skeleton can be divided into the axial skeleton and the appendicular skeleton. The axial skeleton is formed by the vertebral column, the rib cage, the skull and other associated bones. The appendicular skeleton, which is attached to the axial skeleton, is formed by the shoulder girdle, the pelvic girdle and the bones of the upper and lower limbs. The human skeleton performs six major functions: support, movement, protection, production of blood cells, storage of minerals, and endocrine regulation. The human skeleton is not as sexually dimorphic as that of many other primate species, but subtle differenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dental Fluorosis
Dental fluorosis is a common disorder, characterized by hypomineralization of tooth enamel caused by ingestion of excessive fluoride during enamel formation. It appears as a range of visual changes in enamel causing degrees of intrinsic tooth discoloration, and, in some cases, physical damage to the teeth. The severity of the condition is dependent on the dose, duration, and age of the individual during the exposure. The "very mild" (and most common) form of fluorosis, is characterized by small, opaque, "paper white" areas scattered irregularly over the tooth, covering less than 25% of the tooth surface. In the "mild" form of the disease, these mottled patches can involve up to half of the surface area of the teeth. When fluorosis is moderate, all of the surfaces of the teeth are mottled and teeth may be ground down and brown stains frequently "disfigure" the teeth. Severe fluorosis is characterized by brown discoloration and discrete or confluent pitting; brown stains are wid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |