|
Granite
Granite () is a coarse-grained ( phaneritic) intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly cools and solidifies underground. It is common in the continental crust of Earth, where it is found in igneous intrusions. These range in size from dikes only a few centimeters across to batholiths exposed over hundreds of square kilometers. Granite is typical of a larger family of ''granitic rocks'', or '' granitoids'', that are composed mostly of coarse-grained quartz and feldspars in varying proportions. These rocks are classified by the relative percentages of quartz, alkali feldspar, and plagioclase (the QAPF classification), with true granite representing granitic rocks rich in quartz and alkali feldspar. Most granitic rocks also contain mica or amphibole minerals, though a few (known as leucogranites) contain almost no dark minerals. Granite is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Granite Qapf
Granite () is a coarse-grained ( phaneritic) intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly cools and solidifies underground. It is common in the continental crust of Earth, where it is found in igneous intrusions. These range in size from dikes only a few centimeters across to batholiths exposed over hundreds of square kilometers. Granite is typical of a larger family of ''granitic rocks'', or '' granitoids'', that are composed mostly of coarse-grained quartz and feldspars in varying proportions. These rocks are classified by the relative percentages of quartz, alkali feldspar, and plagioclase (the QAPF classification), with true granite representing granitic rocks rich in quartz and alkali feldspar. Most granitic rocks also contain mica or amphibole minerals, though a few (known as leucogranites) contain almost no dark minerals. Granite is nearly a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Igneous Rock
Igneous rock (derived from the Latin word ''ignis'' meaning fire), or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rock is formed through the cooling and solidification of magma or lava. The magma can be derived from partial melts of existing rocks in either a planet's mantle or crust. Typically, the melting is caused by one or more of three processes: an increase in temperature, a decrease in pressure, or a change in composition. Solidification into rock occurs either below the surface as intrusive rocks or on the surface as extrusive rocks. Igneous rock may form with crystallization to form granular, crystalline rocks, or without crystallization to form natural glasses. Igneous rocks occur in a wide range of geological settings: shields, platforms, orogens, basins, large igneous provinces, extended crust and oceanic crust. Geological significance Igneous and metamorphic rocks make up 90–95% of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Batholith
A batholith () is a large mass of intrusive igneous rock (also called plutonic rock), larger than in area, that forms from cooled magma deep in Earth's crust. Batholiths are almost always made mostly of felsic or intermediate rock types, such as granite, quartz monzonite, or diorite (see also ''granite dome''). Formation Although they may appear uniform, batholiths are in fact structures with complex histories and compositions. They are composed of multiple masses, or ''plutons'', bodies of igneous rock of irregular dimensions (typically at least several kilometers) that can be distinguished from adjacent igneous rock by some combination of criteria including age, composition, texture, or mappable structures. Individual plutons are solidified from magma that traveled toward the surface from a zone of partial melting near the base of the Earth's crust. Traditionally, these plutons have been considered to form by ascent of relatively buoyant magma in large masses call ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Granitoid
A granitoid is a generic term for a diverse category of coarse-grained igneous rocks that consist predominantly of quartz, plagioclase, and alkali feldspar. Granitoids range from plagioclase-rich tonalites to alkali-rich syenites and from quartz-poor monzonites to quartz-rich quartzolites. As only two of the three defining mineral groups (quartz, plagioclase, and alkali feldspar) need to be present for the rock to be called a granitoid, foid-bearing rocks, which predominantly contain feldspars but no quartz, are also granitoids. The terms ''granite'' and ''granitic rock'' are often used interchangeably for granitoids; however, granite is just one particular type of granitoid. Granitoids are diverse; no classification system for granitoids can give a complete and unique characterization of the origin, compositional evolution, and geodynamic environment for the genesis of a granitoid. Accordingly, multiple granitoid classification systems have been developed such as those based o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Felsic
In geology, felsic is a modifier describing igneous rocks that are relatively rich in elements that form feldspar and quartz.Marshak, Stephen, 2009, ''Essentials of Geology,'' W. W. Norton & Company, 3rd ed. It is contrasted with mafic rocks, which are relatively richer in magnesium and iron. Felsic refers to silicate minerals, magma, and rocks which are enriched in the lighter elements such as silicon, oxygen, aluminium, sodium, and potassium. Felsic magma or lava is higher in viscosity than mafic magma/lava. Felsic rocks are usually light in color and have specific gravities less than 3. The most common felsic rock is granite. Common felsic minerals include quartz, muscovite, orthoclase, and the sodium-rich plagioclase feldspars (albite-rich). Terminology In modern usage, the term ''acid rock'', although sometimes used as a synonym, normally now refers specifically to a high-silica-content (greater than 63% SiO2 by weight) volcanic rock, such as rhyolite. Older, broader ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magma
Magma () is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natural satellites. Besides molten rock, magma may also contain suspended crystals and gas bubbles. Magma is produced by melting of the mantle or the crust in various tectonic settings, which on Earth include subduction zones, continental rift zones, mid-ocean ridges and hotspots. Mantle and crustal melts migrate upwards through the crust where they are thought to be stored in magma chambers or trans-crustal crystal-rich mush zones. During magma's storage in the crust, its composition may be modified by fractional crystallization, contamination with crustal melts, magma mixing, and degassing. Following its ascent through the crust, magma may feed a volcano and be extruded as lava, or it may solidify underground to form an intrusion, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar. Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at . Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold. There are many different varieties of quartz, several of which are classified as gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia. Quartz is the mineral defining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Granito Nicol Incrociati
Granito (''Granite'') is a city in the state of Pernambuco, Brazil. The population in 2020, according with IBGE was 7,537 inhabitants and the total area is . Geography * State - Pernambuco * Region - Sertão Pernambucano * Boundaries - Exu and Moreilândia (N); Parnamirim (S); Serrita (E); Bodocó (W). * Area - 521.86 km² * Elevation - 447 m * Hydrography - Brigida River * Vegetation - Caatinga * Climate - semi arid - (Sertão) hot * Annual average temperature - 25.4 c * Distance to Recife - 592 km Economy The main economic activities in Granito are based in agribusiness, especially creation of cattle, sheep, goats, donkeys, chickens; and plantations of corn Maize ( ; ''Zea mays'' subsp. ''mays'', from es, maíz after tnq, mahiz), also known as corn (North American and Australian English), is a cereal grain first domesticated by indigenous peoples in southern Mexico about 10,000 years ago. The .... Economic Indicators Economy by Sec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron- endmember annite, and the magnesium-endmember phlogopite; more aluminous end-members include siderophyllite and eastonite. Biotite was regarded as a mineral ''species'' by the International Mineralogical Association until 1998, when its status was changed to a mineral ''group''. The term ''biotite'' is still used to describe unanalysed dark micas in the field. Biotite was named by J.F.L. Hausmann in 1847 in honor of the French physicist Jean-Baptiste Biot, who performed early research into the many optical properties of mica. Members of the biotite group are sheet silicates. Iron, magnesium, aluminium, silicon, oxygen, and hydrogen form sheets that are weakly bound together by potassium ions. The term "iron mica" is sometimes used for iron-rich biotite, but the term also refers to a flaky mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucogranite
Leucogranite is a light-colored, granitic, igneous rock containing almost no dark minerals. Alaskite is a synonym. Leucogranites have been reported from a variety of involving continental collisions. Examples include the ( Trans-Hudson orogeny of |
Phanerite
A phanerite is an igneous rock whose microstructure is made up of crystals large enough to be distinguished with the unaided human eye. In contrast, the crystals in an aphanitic rock are too fine-grained to be identifiable. Phaneritic texture forms when magma deep underground in the pluton In geology, an igneous intrusion (or intrusive body or simply intrusion) is a body of intrusive igneous rock that forms by crystallization of magma slowly cooling below the surface of the Earth. Intrusions have a wide variety of forms and com ...ic environment cools slowly, giving the crystals time to grow. Phanerites are often described as '' coarse-grained'' or '' macroscopically crystalline''. References Phaneritic rocks Petrology {{Petrology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
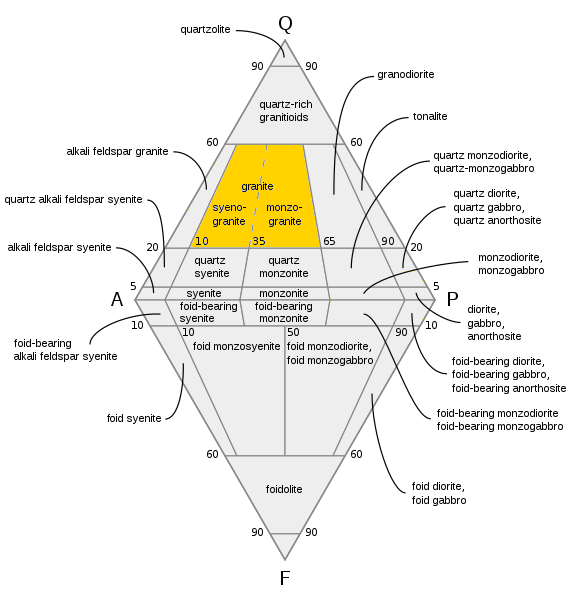
QAPF Diagram
A QAPF diagram is a double ternary diagram which is used to classify igneous rocks based on mineralogic composition. The acronym QAPF stands for " Quartz, Alkali feldspar, Plagioclase, Feldspathoid (Foid)". These are the mineral groups used for classification in QAPF diagram. Q, A, P and F percentages are normalized (recalculated so that their sum is 100%). Origin QAPF diagrams were created by the International Union of Geological Sciences (IUGS): ''Subcommission on the Systematics of Igneous Rocks'' fostered by Albert Streckeisen (whence their alternative name: Streckeisen diagrams). Geologists worldwide accept the diagrams as a classification of igneous, especially plutonic rocks. Usage QAPF diagrams are mostly used to classify plutonic rocks ( phaneritic rocks), but are also used to classify volcanic rocks if modal mineralogical compositions have been determined. QAPF diagrams are not used to classify pyroclastic rocks or volcanic rocks if modal mineralogical compos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |