|
Fluorescent
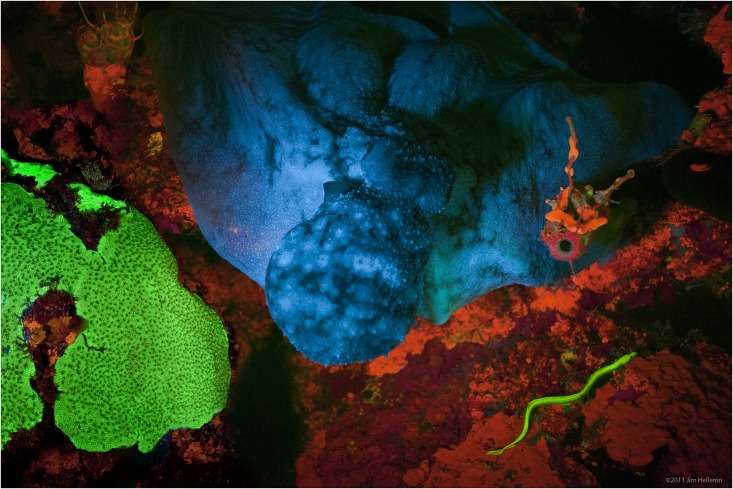
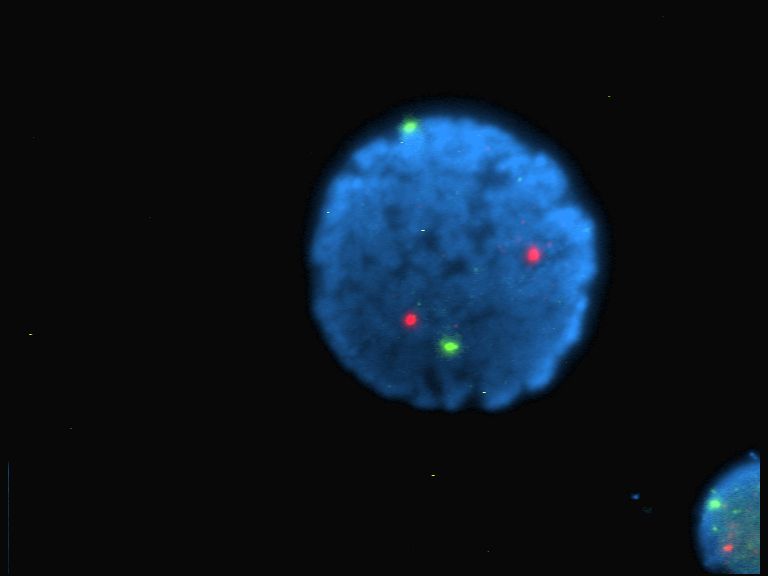
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacuu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the lamp to glow. A fluorescent lamp converts electrical energy into useful light much more efficiently than an incandescent lamp. The typical luminous efficacy of fluorescent lighting systems is 50–100 lumens per watt, several times the efficacy of incandescent bulbs with comparable light output. For comparison, the luminous efficacy of an incandescent bulb may only be 16 lumens per watt. Fluorescent lamp fixtures are more costly than incandescent lamps because, among other things, they require a ballast to regulate current through the lamp, but the initial cost is offset by a much lower running cost. Compact fluorescent lamps are now available in the same popular sizes as incand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Minerals Hg
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacuu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Labelling
In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Generally, fluorescence, fluorescent tagging, or labeling, uses a reactive derivative of a fluorescent molecule known as a fluorophore. The fluorophore selectively binds to a specific region or functional group on the target molecule and can be attached chemically or biologically. Various labeling techniques such as enzymatic labeling, protein tag, protein labeling, and genetic labeling are widely utilized. Ethidium bromide, fluorescein and green fluorescent protein are common tags. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target. History The development of methods to detect and identify biomolecules has been motivated by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. Fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
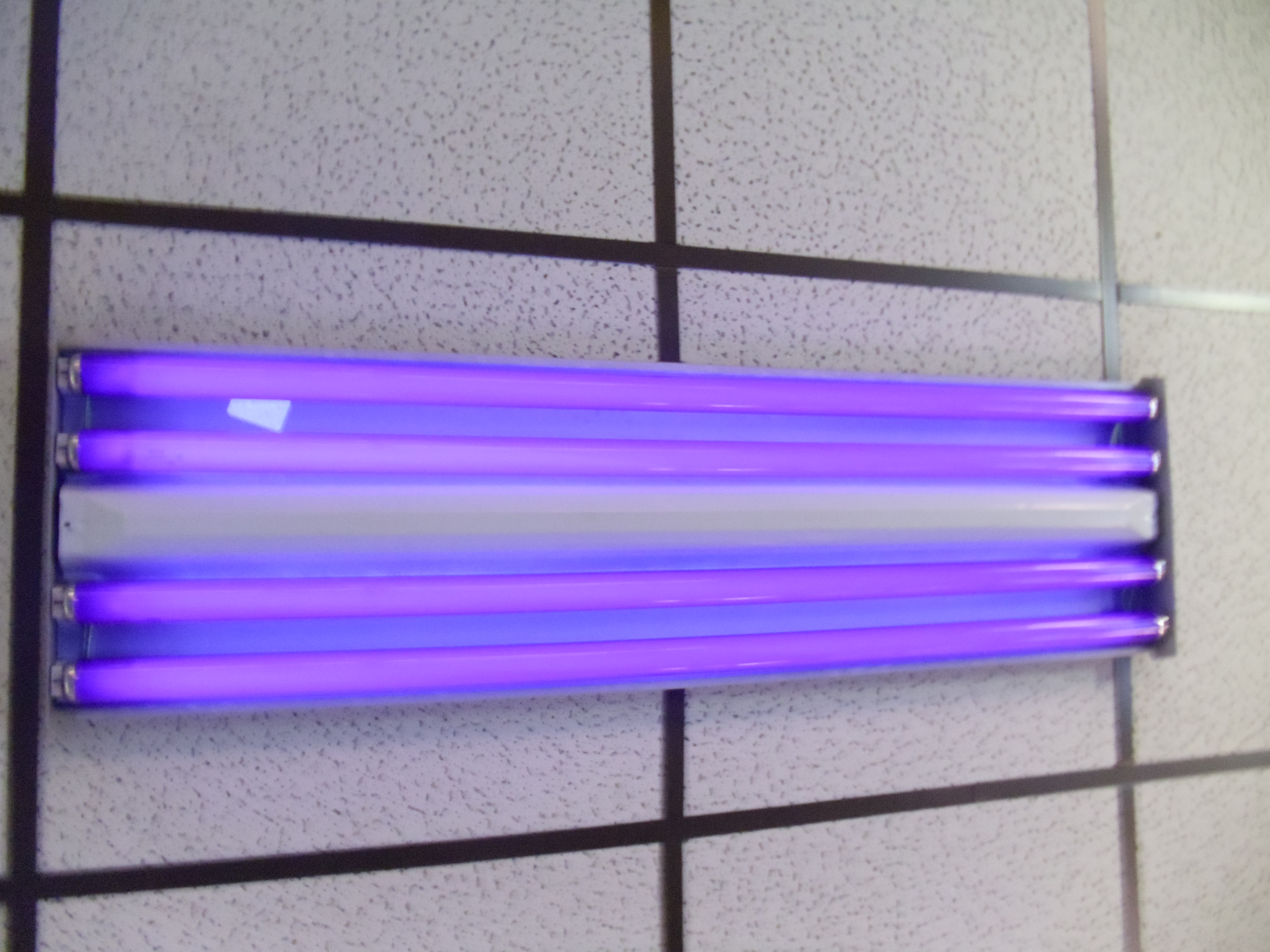
Blacklight
A blacklight, also called a UV-A light, Wood's lamp, or ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and very little visible light. One type of lamp has a violet filter material, either on the bulb or in a separate glass filter in the lamp housing, which blocks most visible light and allows through UV, so the lamp has a dim violet glow when operating. Blacklight lamps which have this filter have a lighting industry designation that includes the letters "BLB". This stands for "blacklight blue". A second type of lamp produces ultraviolet but does not have the filter material, so it produces more visible light and has a blue color when operating. These tubes are made for use in "bug zapper" insect traps, and are identified by the industry designation "BL". This stands for "blacklight". Blacklight sources may be specially designed fluorescent lamps, mercury-vapor lamps, light-emitting diodes (LEDs), lasers, or incandescent lamps. In medicine, for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Fluorescent Display
A vacuum fluorescent display (VFD) is a display device once commonly used on consumer electronics equipment such as video cassette recorders, car radios, and microwave ovens. A VFD operates on the principle of cathodoluminescence, roughly similar to a cathode ray tube, but operating at much lower voltages. Each tube in a VFD has a phosphor-coated carbon anode that is bombarded by electrons emitted from the cathode filament.Chen, J., Cranton, W., & Fihn, M. (Eds.). (2016). Handbook of Visual Display Technology. doi:10.1007/978-3-319-14346-0 page 1610 onwards In fact, each tube in a VFD is a triode vacuum tube because it also has a mesh control grid. Unlike liquid crystal displays, a VFD emits very bright light with high contrast and can support display elements of various colors. Standard illumination figures for VFDs are around 640 cd/m2 with high-brightness VFDs operating at 4,000 cd/m2, and experimental units as high as 35,000 cd/m2 depending on the drive vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas-discharge Lamp
Gas-discharge lamps are a family of artificial light sources that generate light by sending an electric discharge through an ionized gas, a plasma. Typically, such lamps use a noble gas (argon, neon, krypton, and xenon) or a mixture of these gases. Some include additional substances, like mercury, sodium, and metal halides, which are vaporized during startup to become part of the gas mixture. Single ended self-starting lamps are insulated with a mica disc and contained in a borosilicate glass gas discharge tube (arc tube) and a metal cap. They include the sodium-vapor lamp that is the gas-discharge lamp in street lighting. In operation, some of the electrons are forced to leave the atoms of the gas near the anode by the electric field applied between the two electrodes, leaving these atoms positively ionized. The free electrons thus released flow onto the anode, while the cations thus formed are accelerated by the electric field and flow towards the cathode. Typicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LED Lamps
An LED lamp or LED light bulb is an electric light that produces light using light-emitting diodes (LEDs). LED lamps are significantly more energy-efficient than equivalent incandescent lamps and can be significantly more efficient than most fluorescent lamps. The most efficient commercially available LED lamps have efficiencies of 200 lumen per watt (Lm/W). Commercial LED lamps have a lifespan many times longer than incandescent lamps. LED lamps require an electronic LED driver circuit to operate from mains power lines, and losses from this circuit means that the efficiency of the lamp is lower than the efficiency of the LED chips it uses. The driver circuit may require special features to be compatible with lamp dimmers intended for use on incandescent lamps. Generally the current waveform contains some amount of distortion, depending on the luminaires’ technology. The LED lamp market is projected to grow from US$75.8 billion in 2020 and increasing to US$160 billion in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incandescent Lamp
An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxidation. Current is supplied to the filament by terminals or wires embedded in the glass. A bulb socket provides mechanical support and electrical connections. Incandescent bulbs are manufactured in a wide range of sizes, light output, and voltage ratings, from 1.5 volts to about 300 volts. They require no external regulating equipment, have low manufacturing costs, and work equally well on either alternating current or direct current. As a result, the incandescent bulb became widely used in household and commercial lighting, for portable lighting such as table lamps, car headlamps, and flashlights, and for decorative and advertising lighting. Incandescent bulbs are much less efficient than other types of electric lighting, converting le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
White
White is the lightest color and is achromatic (having no hue). It is the color of objects such as snow, chalk, and milk, and is the opposite of black. White objects fully reflect and scatter all the visible wavelengths of light. White on television and computer screens is created by a mixture of red, blue, and green light. The color white can be given with white pigments, especially titanium dioxide. In ancient Egypt and ancient Rome, priestesses wore white as a symbol of purity, and Romans wore white togas as symbols of citizenship. In the Middle Ages and Renaissance a white unicorn symbolized chastity, and a white lamb sacrifice and purity. It was the royal color of the kings of France, and of the monarchist movement that opposed the Bolsheviks during the Russian Civil War (1917–1922). Greek and Roman temples were faced with white marble, and beginning in the 18th century, with the advent of neoclassical architecture, white became the most common color of new churches ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode-ray Tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms (oscilloscope), pictures (television set, computer monitor), radar targets, or other phenomena. A CRT on a television set is commonly called a picture tube. CRTs have also been used as memory devices, in which case the screen is not intended to be visible to an observer. The term ''cathode ray'' was used to describe electron beams when they were first discovered, before it was understood that what was emitted from the cathode was a beam of electrons. In CRT television sets and computer monitors, the entire front area of the tube is scanned repeatedly and systematically in a fixed pattern called a raster. In color devices, an image is produced by controlling the intensity of each of three electron beams, one for each additive primary color (red, green, and blue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |