|
Flame Polishing
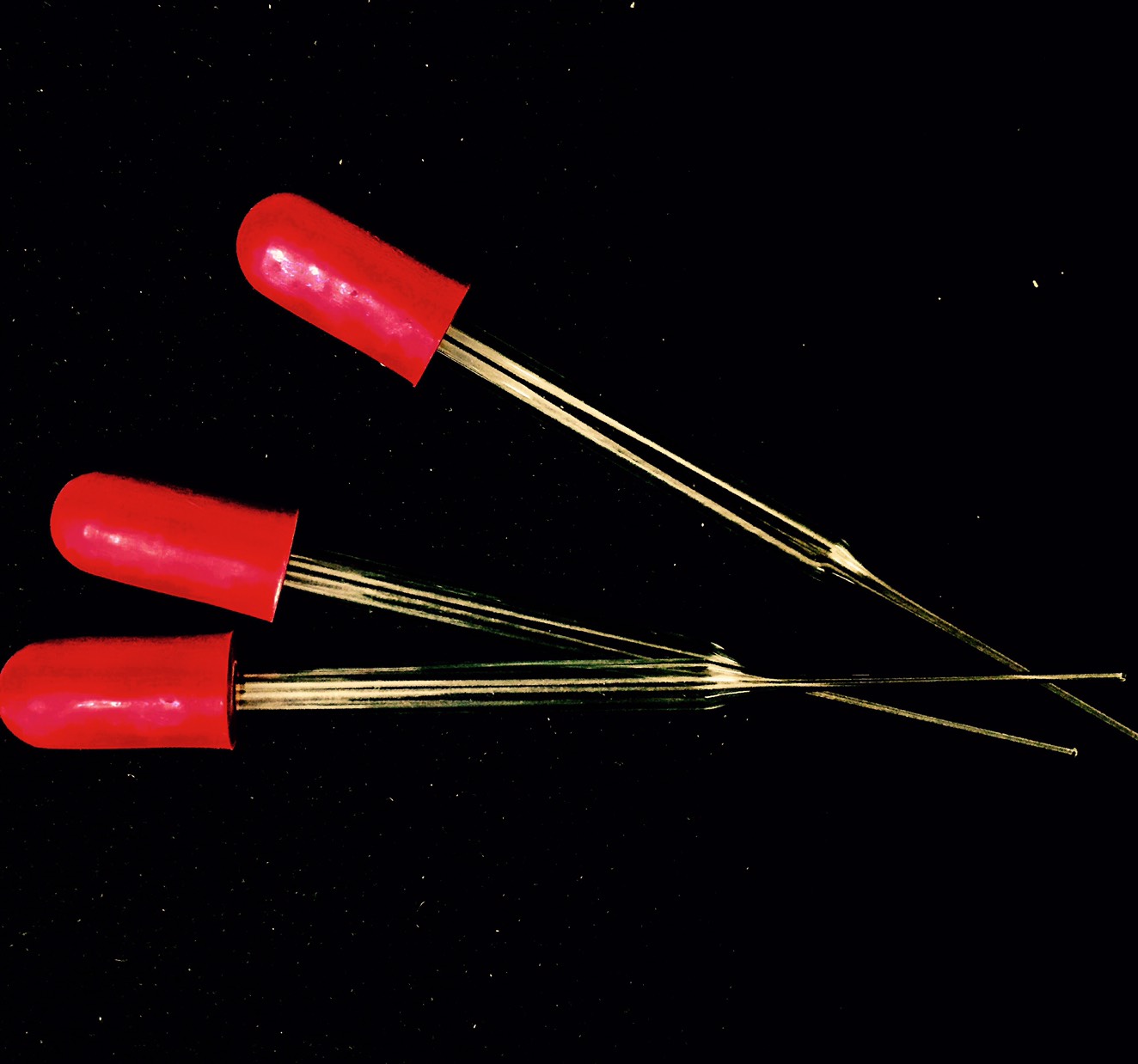
Fire polishing, also known as flame polishing, is a method of polishing a material, usually glass or thermoplastics, by exposing it to a flame or heat. When the surface of the material briefly melts, surface tension smooths the surface. Operator skill is critical with this method. When done properly, flame plastic polishing produces the clearest finish, especially when polishing acrylic. This method is most applicable to flat external surfaces. Flame polishing is frequently used in acrylic plastic fabrication because of its high speed compared to abrasive methods. In this application, an oxyhydrogen torch is typically used, one reason being that the flame chemistry is unlikely to contaminate the plastic. Flame polishing is essential to creation of the glass pipettes used for the patch clamp technique of voltage clamping. Equipment Various machines and torches/gas burners are used in the flame polishing process. Depending on the heating requirements for an intended application, d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polishing
Polishing is the process of creating a smooth and shiny surface by rubbing it or by applying a chemical treatment, leaving a clean surface with a significant specular reflection (still limited by the index of refraction of the material according to the Fresnel equations). In some materials (such as metals, glasses, black or transparent stones), polishing is also able to reduce diffuse reflection to minimal values. When an unpolished surface is magnified thousands of times, it usually looks like a succession of mountains and valleys. By repeated abrasion, those "mountains" are worn down until they are flat or just small "hills." The process of polishing with abrasives starts with a coarse grain size and gradually proceeds to the finer ones to efficiently flatten the surface imperfections and to obtain optimal results. Mechanical properties The strength of polished products can be higher than their unpolished counterparts owing to the removal of stress concentrations prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling ( quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material. D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoplastic
A thermoplastic, or thermosoft plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling. Most thermoplastics have a high molecular weight. The polymer chains associate by intermolecular forces, which weaken rapidly with increased temperature, yielding a viscous liquid. In this state, thermoplastics may be reshaped and are typically used to produce parts by various polymer processing techniques such as injection molding, compression molding, calendering, and extrusion. Thermoplastics differ from thermosetting polymers (or "thermosets"), which form irreversible chemical bonds during the curing process. Thermosets do not melt when heated, but typically decompose and do not reform upon cooling. Above its glass transition temperature and below its melting point, the physical properties of a thermoplastic change drastically without an associated phase change. Some thermoplastics do not fully crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame
A flame (from Latin '' flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they are then considered plasma. Mechanism Color and temperature of a flame are dependent on the type of fuel involved in the combustion, as, for example, when a lighter is held to a candle. The applied heat causes the fuel molecules in the candle wax to vaporize (if this process happens in inert atmosphere without oxidizer, it is called pyrolysis). In this state they can then readily react with oxygen in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to decompose, forming various incomplete combustion products and free radicals, and these products then react with each other and wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane is dependent on the amount of deformation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylic Resin
186 px, Polyhydroxyethylmethacrylate is a typical acrylate resin. An acrylic resin is a thermoplastic or thermosetting plastic substance typically derived from acrylic acid, methacrylic acid and acrylate monomers such as butyl acrylate and or methacrylate monomers such as methyl methacrylate. Thermoplastic acrylics designate a group of acrylic resins typically containing both a high molecular weight and a high glass transition temperature which exhibit lacquer dry capability. Acrylic resins designed for use in two component systems for crosslinking with isocyanate are referred to as polyols and are made with the monomers previously mentioned as well as hydroxy monomers such as hydroxy ethyl methacrylate. Acrylic resins are produced in different liquid carriers such as a hydrocarbon solvent (solventborne acrylics or solution acrylics solventborne acrylic selector) or water in which case they are referred to as emulsions or dispersions and they are also provided in 100% solids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyhydrogen Torch
Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough to achieve maximum efficiency; in practice a ratio 4:1 or 5:1 is needed to avoid an oxidizing flame. This mixture may also be referred to as ' (Scandinavian and German Knallgas: "bang-gas"), although some authors define knallgas to be a generic term for the mixture of fuel with the precise amount of oxygen required for complete combustion, thus 2:1 oxyhydrogen would be called "hydrogen-knallgas". "Brown's gas" and HHO are terms for oxyhydrogen mainly encountered in fringe science. Properties Oxyhydrogen will combust when brought to its autoignition temperature. For the stoichiometric mixture, 2:1 hydrogen:oxygen, at normal atmospheric pressure, autoignition occurs at about 570 °C (1065 °F). The minimum energy required ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pasteur Pipette
An eye dropper, also called Pasteur pipette or simply dropper, is a device used to transfer small quantities of liquids. They are used in the laboratory and also to dispense small amounts of liquid medicines. A very common use was to dispense eye drops into the eye. The commonly recognized form is a glass tube tapered to a narrow point (a pipette) and fitted with a rubber bulb at the top, although many styles of both plastic and glass droppers exist. The combination of the pipette and rubber bulb has also been referred to as a teat pipette. The ''Pasteur pipette'' name is from the French scientist Louis Pasteur, who used a variant of them extensively during his research. In the past, there was no equipment to transfer a chemical solution without exposing it to the external environment. The hygiene and purity of chemical compounds is necessary for the expected result of each experiment. The eye dropper, both glass and plastic types, can be sterilized and plugged with a rubber bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patch Clamp
The patch clamp technique is a laboratory technique in electrophysiology used to study ionic currents in individual isolated living cells, tissue sections, or patches of cell membrane. The technique is especially useful in the study of excitable cells such as neurons, cardiomyocytes, muscle fibers, and pancreatic beta cells, and can also be applied to the study of bacterial ion channels in specially prepared giant spheroplasts. Patch clamping can be performed using the voltage clamp technique. In this case, the voltage across the cell membrane is controlled by the experimenter and the resulting currents are recorded. Alternatively, the current clamp technique can be used. In this case, the current passing across the membrane is controlled by the experimenter and the resulting changes in voltage are recorded, generally in the form of action potentials. Erwin Neher and Bert Sakmann developed the patch clamp in the late 1970s and early 1980s. This discovery made it possib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage Clamp
The voltage clamp is an experimental method used by electrophysiologists to measure the ion currents through the membranes of excitable cells, such as neurons, while holding the membrane voltage at a set level. A basic voltage clamp will iteratively measure the membrane potential, and then change the membrane potential (voltage) to a desired value by adding the necessary current. This "clamps" the cell membrane at a desired constant voltage, allowing the voltage clamp to record what currents are delivered. Because the currents applied to the cell must be equal to (and opposite in charge to) the current going across the cell membrane at the set voltage, the recorded currents indicate how the cell reacts to changes in membrane potential. Cell membranes of excitable cells contain many different kinds of ion channels, some of which are voltage-gated. The voltage clamp allows the membrane voltage to be manipulated independently of the ionic currents, allowing the current–voltage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Burner
A gas burner is a device that produces a controlled flame by mixing a fuel gas such as acetylene, natural gas, or propane with an oxidizer such as the ambient air or supplied oxygen, and allowing for ignition and combustion. The flame is generally used for the heat, infrared radiation, or visible light it produces. Some burners, such as gas flares, dispose of unwanted or uncontainable flammable gases. Some burners are operated to produce carbon black. The gas burner has many applications such as soldering, brazing, and welding, the latter using oxygen instead of air for producing a hotter flame, which is required for melting steel. Chemistry laboratories use natural-gas fueled Bunsen burners. In domestic and commercial settings gas burners are commonly used in gas stoves and cooktops. For melting metals with melting points of up to 1100 °C (such as copper, silver, and gold Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Hardening
Fire hardening, also known as "fire-danubing", is the process of removing moisture from wood, changing its structure and material properties, by charring it over or directly in a fire or a bed of coals. This has been thought to make a point, like that of a spear or arrow, or an edge, like that of a knife or axe, more durable and efficient for its use as a tool or weapon. An initial study suggests that the process might make the wood brittle but would substantially reduce the time needed to make a spear point. Fire hardening may be done before, after, or during the manufacturing of the wooden tip. Longer procedures involving greasing and polishing with stones to impregnate the wood with fats and oils and silica may improve the effects of the process. Fire hardening was first developed by primitive humans at least 400,000 years ago—long before flint or stone points. See also * Wood working * Flame polishing Fire polishing, also known as flame polishing, is a method of polishing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |