|
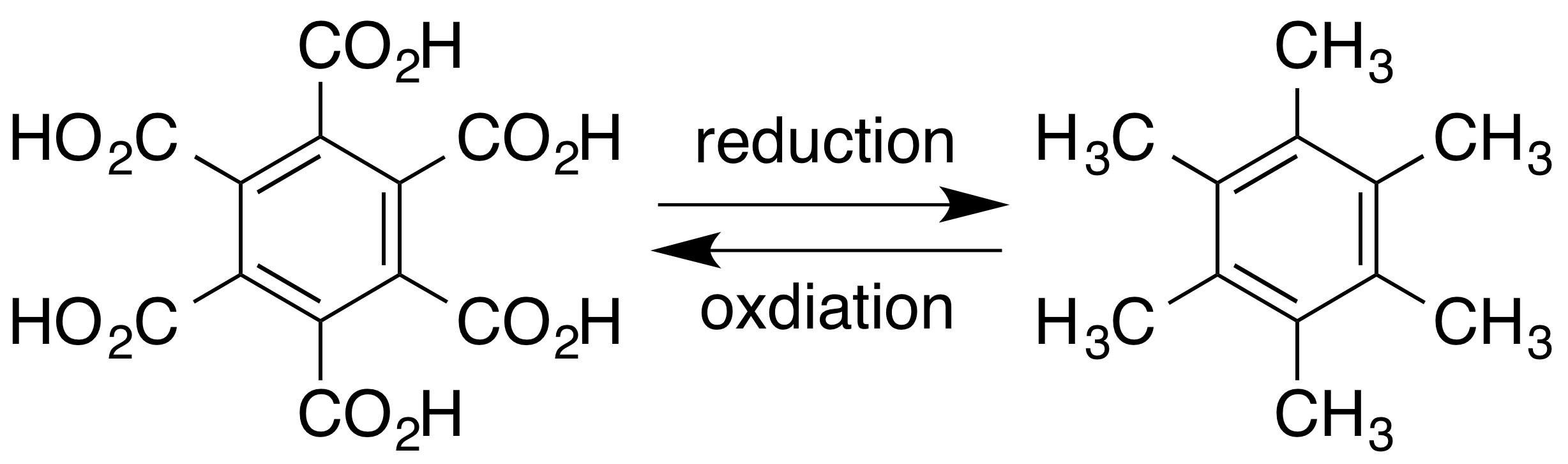
Durene
Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry. Production It is a component of coal tar and was first prepared from pseudocumene in 1870. It is produced by methylation of other methylated benzene compounds such as ''p''-xylene and pseudocumene. :C6H4(CH3)2 + 2 CH3Cl → C6H2(CH3)4 + 2 HCl In industry, a mixture of xylenes and trimethylbenzenes is alkylated with methanol. Durene can be separated from its isomers by selective crystallization, exploiting its high melting point. The original synthesis of durene involved a similar reaction starting from toluene. Durene is a significant bypr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isodurene
Isodurene or 1,2,3,5-tetramethylbenzene is an organic compound with the formula C6H2(CH3)4, classified as an aromatic hydrocarbon. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar. Isodurene is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene). Preparation Isoodurene can be prepared from mesitylene, which is converted to mesityl bromide. The latter reacts with magnesium to give the Grignard reagent, which can be alkylated with dimethyl sulfate: Industrially, isodurene can be isolated from the reformed fraction of oil refineries. It may also be produced by methylation of toluene, xylenes, and trimethylbenzenes The trimethylbenzenes constitute a group of substances of aromatic hydrocarbons, which structure consists of a benzene ring with three methyl groups (–CH3) as a substituent. Throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prehnitene
Prehnitene or 1,2,3,4-tetramethylbenzene is an organic compound with the formula C6H2(CH3)4, classified as an aromatic hydrocarbon. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar. Prehnitene is one of three isomers of tetramethylbenzene, the other two being isodurene (1,2,3,5-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene). It is a relatively easily oxidized benzene derivative, with E1/2 of 2.0 V vs NHE. Production Industrially, prehnitene can be isolated from the reformed fraction of oil refineries. It may also be produced by methylation of toluene, xylenes and the trimethylbenzenes hemimellitene and pseudocumene 1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula CH(CH). Classified as an aromatic hydrocarbon, it is a flammable colorless liquid with a strong odor. It is nearly insoluble in water but soluble .... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethylbenzene
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929 Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity. The compound can be prepared by reacting phenol with methanol at elevated temperatures over a suitable solid catalyst such as alumina. The mechanism of the process has been studied extensively, with several intermediates having been identified. Alkyne trimerisation of dimethylacetylene also yields hexamethylbenzene in the presence of a suitable catalyst. Hexamet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas To Liquids
Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive. The motivation for GTL is to produce liquid fuels, which are more readily transported than methane. Methane must be cooled below its critical temperature of -82.3 °C in order to be liquified under pressure. Because of the associated cryogenic apparatus, LNG tank ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duroquinone
Duroquinone is an organic oxidant (C6(CH3)4O2). It is related to 1,4-benzoquinone by replacement of four H centres with methyl (Me) groups. The C10O2 core of this molecule is planar with two pairs of C=O and C=C bonds.J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Quinones as Electron Acceptors. X-Ray Structures, Spectral (EPR, UV-vis) Characteristics and Electron-Transfer Reactivities of Their Reduced Anion Radicals as Separated vs Contact Ion Pairs" Journal of the American Chemical Society 2006 128, 16708-16719. The compound is produced via nitration of durene (1,2,4,5-tetramethylbenzene) followed reduction to the diamine and then oxidation. A derived organoiron compound (η2,η2-C6(CH3)4O2)Fe(CO)3 is obtained by the carbonylation of 2-butyne in the presence of iron pentacarbonyl Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon Solvents
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon dio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arkivoc
''Arkivoc'' (''Archive for Organic Chemistry'') is a peer-reviewed open access scientific journal covering all aspects of organic chemistry. It is published by the non-profit organization Arkat USA, which was established in 2000 through a personal donation from Alan R. Katritzky and Linde Katritzky. ''Arkivoc'' is the primary publication of Arkat USA. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 1.165, ranking it 37th out of 57 journals in the category "Chemistry, Organic". Abstracting and Indexing According to the Journal Citation Reports, the journal has a 2018 impact factor of 1.253. The journal is indexed in Web of Science: Science Citation Index Expanded. References External link ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irina P
Irina ( Cyrillic: Ирина) is a feminine given name of Ancient Greek origin, commonly borne by followers of the Eastern Orthodox Church. It is derived from Eirene (Ancient Greek: Εἰρήνη), an ancient Greek goddess, personification of peace. It is mostly used in countries within the Commonwealth of Independent States and the Balkans. Diminutive forms in Slavic languages include Ira, Irinka, Irinushka, Irisha, Irka, Irochka, Irinochka. Origin Irina is connected with Irene of Macedonia who was the first woman recognized by the church as a great martyr. She was born pagan as Penelope and later baptized as Irene. Some sources refer to her being baptized by Saint Timothy, in which case she lived in the 1st–2nd century, while others date her death in the year 315. Opinions also differ about the location of her birthplace, the city of Magedon, placing it either in Persia or in Migdonia ( Macedonia). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Standard
An internal standard in analytical chemistry is a chemical substance that is added in a constant amount to samples, the blank and calibration standards in a chemical analysis. This substance can then be used for calibration by plotting the ratio of the analyte signal to the internal standard signal as a function of the analyte concentration of the standards. This is done to correct for the loss of analyte during sample preparation or sample inlet. The internal standard is a compound that is very similar, but not identical to the chemical species of interest in the samples, as the effects of sample preparation should, relative to the amount of each species, be the same for the signal from the internal standard as for the signal(s) from the species of interest in the ideal case. Adding known quantities of analyte(s) of interest is a distinct technique called standard addition, which is performed to correct for matrix effects. This ratio for the samples is then used to obtain their anal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H (hydrogen-1; i.e. having a proton for a nucleus). Simple NMR spectra are recorded in solution, and solvent protons must not be allowed to interfere. Deuterated (deuterium = 2H, often symbolized as D) solvents especially for use in NMR are preferred, e.g. deuterated water, D2O, deuterated acetone, (CD3)2CO, deuterated methanol, CD3OD, deuterated dimethyl sulfoxide, (CD3)2SO, and deuterated chloroform, CDCl3. However, a solvent without hydrogen, such as carbon tetrachloride, CCl4 or carbon disulfide, CS2, may also be used. Historically, deuterated solvents were supplied with a small amount (typically 0.1%) of tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on naturally occurring resins. Plants secrete resins for their protective benefits in response to injury. The resin protects the plant from insects and pathogens. Resins confound a wide range of herbivores, insects, and pathogens, while the volatile phenolic compounds may attract benefactors such as parasitoids or predators of the herbivores that attack the plant. Composition Most plant resins are composed of terpenes. Specific components are alpha-pinene, beta-pinene, delta-3 carene, and sabinene, the monocyclic terpenes limonene and terpinolene, and smaller amounts of the tricyclic sesquiterpenes, longifolene, caryophyllene, and delta-cadinene. Some resins also contain a high proportion of resin acids. Rosins on the other hand are less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)