|
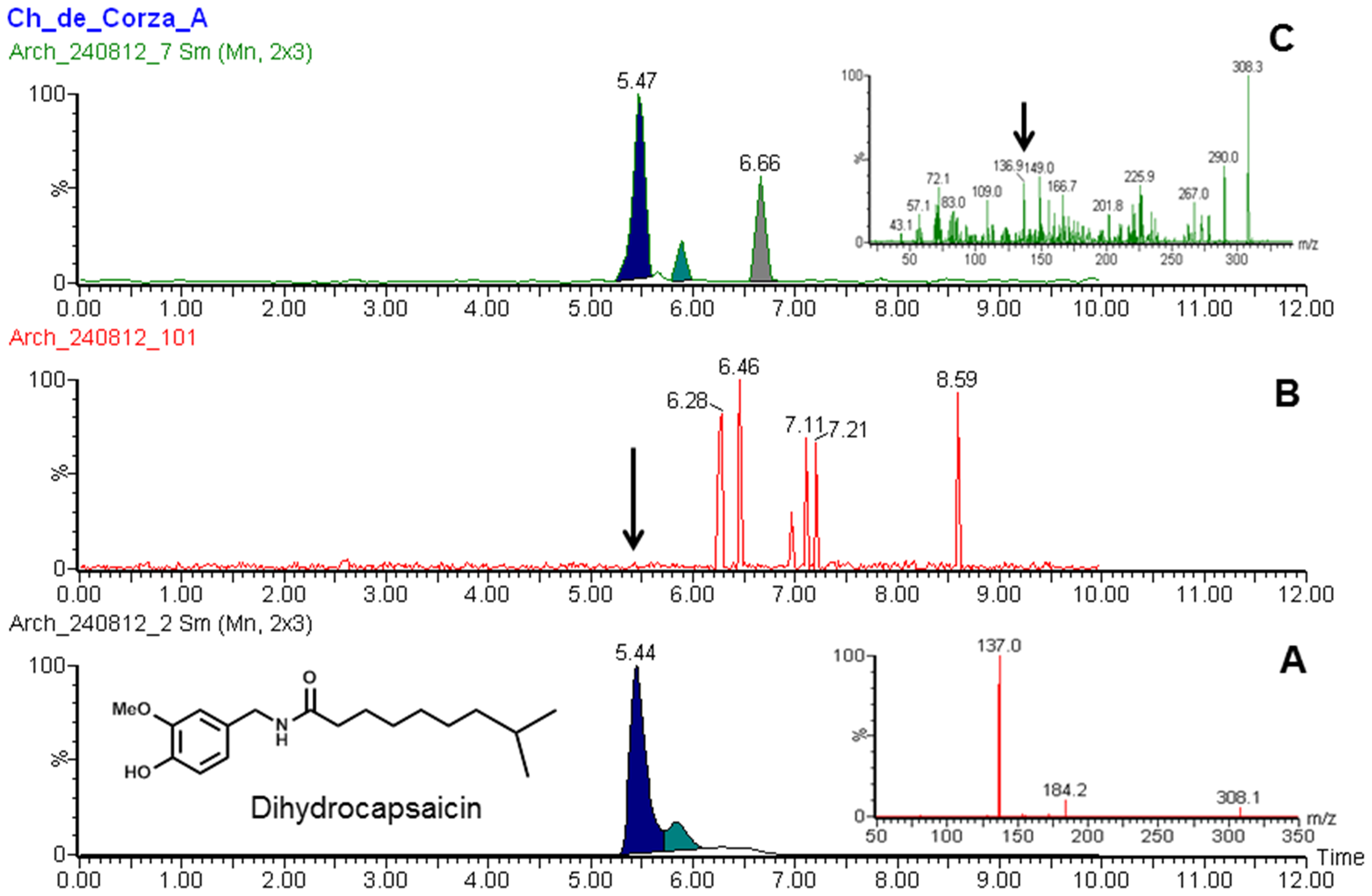
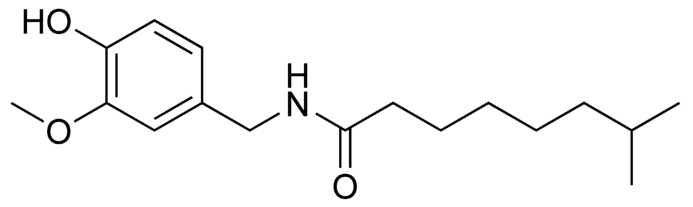
Dihydrocapsaicin
Dihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin, it is an irritant. It accounts for about 22% of the total capsaicinoid mixture and has the same pungency as capsaicin. Pure dihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It is soluble in dimethyl sulfoxide and 100% ethanol. See also * Capsaicin * Nordihydrocapsaicin * Homocapsaicin * Homodihydrocapsaicin * Nonivamide * Scoville scale * Pepper spray * Hot sauce Hot sauce is a type of condiment, seasoning, or salsa made from chili peppers and other ingredients. Many commercial varieties of mass-produced hot sauce exist. History Humans have used chili peppers and other hot spices for thousands of ye ... References External links Molecule of the MonthSafety MSDS data {{Transient receptor potential channel modulators Capsaicinoids Acetamides Methoxy compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrocapsaicin UPLC MS-MS Journal
Dihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin, it is an irritant. It accounts for about 22% of the total capsaicinoid mixture and has the same pungency as capsaicin. Pure dihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It is soluble in dimethyl sulfoxide and 100% ethanol. See also * Capsaicin * Nordihydrocapsaicin * Homocapsaicin * Homodihydrocapsaicin * Nonivamide * Scoville scale * Pepper spray * Hot sauce Hot sauce is a type of condiment, seasoning, or salsa made from chili peppers and other ingredients. Many commercial varieties of mass-produced hot sauce exist. History Humans have used chili peppers and other hot spices for thousands of ye ... References External links Molecule of the MonthSafety MSDS data {{Transient receptor potential channel modulators Capsaicinoids Acetamides Methoxy compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scoville Scale
The Scoville scale is a measurement of the pungency (spiciness or "heat") of chili peppers, as recorded in Scoville heat units (SHU), based on the concentration of capsaicinoids, among which capsaicin is the predominant component. The scale is named after its creator, American pharmacist Wilbur Scoville, whose 1912 method is known as the Scoville organoleptic test. The Scoville organoleptic test is a subjective assessment derived from the capsaicinoid sensitivity by people experienced with eating hot chilis. An alternative method, high-performance liquid chromatography (HPLC), can be used to analytically quantify the capsaicinoid content as an indicator of pungency. As of 2011, the subjective organoleptic test has been largely superseded by analytical methods such as HPLC. Scoville organoleptic test In the Scoville organoleptic test, an exact weight of dried pepper is dissolved in alcohol to extract the heat components (capsaicinoids), then diluted in a solution of suga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsaicinoids
Capsaicin (8-methyl-''N''-vanillyl-6-nonenamide) ( or ) is an active component of chili peppers, which are plants belonging to the genus ''Capsicum''. It is a chemical irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related alkaloids are called capsaicinoids and are produced as secondary metabolites by chili peppers, probably as deterrents against certain mammals and fungi.What Made Chili Peppers So Spicy? Talk of the Nation, 15 August 2008. Pure capsaicin is a , colorless, highly |
Capsaicin
Capsaicin (8-methyl-''N''-vanillyl-6-nonenamide) ( or ) is an active component of chili peppers, which are plants belonging to the genus ''Capsicum''. It is a chemical irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related alkaloids are called capsaicinoids and are produced as secondary metabolites by chili peppers, probably as deterrents against certain mammals and fungi.What Made Chili Peppers So Spicy? Talk of the Nation, 15 August 2008. Pure capsaicin is a hydrophobic, colorless, highly pungent, crystalline to waxy s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsaicin
Capsaicin (8-methyl-''N''-vanillyl-6-nonenamide) ( or ) is an active component of chili peppers, which are plants belonging to the genus ''Capsicum''. It is a chemical irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related alkaloids are called capsaicinoids and are produced as secondary metabolites by chili peppers, probably as deterrents against certain mammals and fungi.What Made Chili Peppers So Spicy? Talk of the Nation, 15 August 2008. Pure capsaicin is a hydrophobic, colorless, highly pungent, crystalline to waxy s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nordihydrocapsaicin
Nordihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Properties Like capsaicin, it is an irritant. Nordihydrocapsaicin accounts for about 7% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure nordihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy solid. On the Scoville scale it has 9,100,000 SHU (''Scoville heat units''), significantly higher than pepper spray. See also * Capsaicin * Dihydrocapsaicin * Homocapsaicin * Homodihydrocapsaicin * Nonivamide * Scoville scale * Pepper spray * Spice A spice is a seed, fruit, root, bark, or other plant substance primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of plants used for flavoring or as a garnish. Spice ... References {{Transient receptor potential channel modulators Capsaicinoids Acetamides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homocapsaicin
Homocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin it is an irritant. Homocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. On the Scoville scale it has 8,600,000 SHU (''Scoville heat units''). Homocapsaicin isolated from chili pepper has been found in two isomeric forms, both with a carbon-carbon double bond at the 6 position (numbered from the amide carbon) on the 10-carbon acyl chain. One isomer has an additional carbon, a methyl group, at the 8 position and the other has a methyl group at the 9 position. Homocapsaicin (6-ene-8-methyl) is the more abundant isomer. Homocapsaicin with the double bond at the 7 position has never been found in nature, though its structure is widely reported on the Internet and in the scientific literature. Details of this misidentificati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodihydrocapsaicin
Homodihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (''Capsicum''). Like capsaicin it is an irritant. Homodihydrocapsaicin accounts for about 1% of the total capsaicinoids mixture and has about half the pungency of capsaicin. Pure homodihydrocapsaicin is a lipophilic colorless odorless crystalline to waxy compound. It produces "numbing burn" in the throat and is one of the most prolonged and difficult to rinse out. On the Scoville scale it has 8,600,000 SHU (Scoville heat units). See also * Capsaicin * Dihydrocapsaicin * Nordihydrocapsaicin * Homocapsaicin * Nonivamide * Scoville scale * Pepper spray * Spice A spice is a seed, fruit, root, bark, or other plant substance primarily used for flavoring or coloring food. Spices are distinguished from herbs, which are the leaves, flowers, or stems of plants used for flavoring or as a garnish. Spices a ... References External links Molecule of the Month {{Transient recepto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsicum
''Capsicum'' () is a genus of flowering plants in the nightshade family Solanaceae, native to the Americas, cultivated worldwide for their chili pepper or bell pepper fruit. Etymology and names The generic name may come from Latin , meaning 'box', presumably alluding to the pods; or possibly from the Greek word , 'to gulp'. The name "pepper" comes from the similarity of piquance (spiciness or "heat") of the flavor to that of black pepper, '' Piper nigrum'', although there is no botanical relationship with it or with Sichuan pepper. The original term, ''chilli'' (now ''chile'' in Mexico) came from the Nahuatl word ''chīlli'', denoting a larger ''Capsicum'' variety cultivated at least since 3000 BC, as evidenced by remains found in pottery from Puebla and Oaxaca. Different varieties were cultivated in South America, where they are known as ''ajíes'' (singular ''ají''), from the Quechua term for ''Capsicum''. The fruit (technically berries in the strict botanic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irritation
Irritation, in biology and physiology, is a state of inflammation or painful reaction to allergy or cell-lining damage. A stimulus or agent which induces the state of irritation is an irritant. Irritants are typically thought of as chemical agents (for example phenol and capsaicin) but mechanical, thermal (heat), and radiative stimuli (for example ultraviolet light or ionising radiations) can also be irritants. Irritation also has non-clinical usages referring to bothersome physical or psychological pain or discomfort. Irritation can also be induced by some allergic response due to exposure of some allergens for example contact dermatitis, irritation of mucosal membranes and pruritus. Mucosal membrane is the most common site of irritation because it contains secretory glands that release mucous which attracts the allergens due to its sticky nature. Chronic irritation is a medical term signifying that afflictive health conditions have been present for a while. There are many dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipophilic (translated as "fat-loving" or "fat-liking"), and the axiom that "like dissolves like" generally holds true. Thus lipophilic substances tend to dissolve in other lipophilic substances, but hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances. Lipophilicity, hydrophobicity, and non-polarity may describe the same tendency towards participation in the London dispersion force, as the terms are often used interchangeably. However, the terms "lipophilic" and "hydrophobic" are not synonymous, as can be seen with silicones and fluorocarbons, which are hydrophobic but not lipophilic. __TOC__ Surfactants Hydrocarbon-based surfactants are compounds that are amphiphilic (or amphipathic), having ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of the K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |