|
Crystallite
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a ( single) crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in-between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Texture (chemistry)
In physical chemistry and materials science, texture is the distribution of crystallographic orientations of a polycrystalline sample (it is also part of the geological fabric). A sample in which these orientations are fully random is said to have no distinct texture. If the crystallographic orientations are not random, but have some preferred orientation, then the sample has a weak, moderate or strong texture. The degree is dependent on the percentage of crystals having the preferred orientation. Texture is seen in almost all engineered materials, and can have a great influence on materials properties. The texture forms in materials during thermo-mechanical processes, for example during production processes e.g. rolling. Consequently, the rolling process is often followed by a heat treatment to reduce the amount of unwanted texture. Controlling the production process in combination with the characterization of texture and the material's microstructure help to determine the materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grain Boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional defects in the crystal structure, and tend to decrease the electrical and thermal conductivity of the material. Most grain boundaries are preferred sites for the onset of corrosion and for the precipitation of new phases from the solid. They are also important to many of the mechanisms of creep. On the other hand, grain boundaries disrupt the motion of dislocations through a material, so reducing crystallite size is a common way to improve mechanical strength, as described by the Hall–Petch relationship. High and low angle boundaries It is convenient to categorize grain boundaries according to the extent of misorientation between the two grains. ''Low-angle grain boundaries'' (''LAGB'') or ''subgrain boundaries'' are those with a misorientation less than about 15 degrees. Generally speaking they are composed of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among variou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
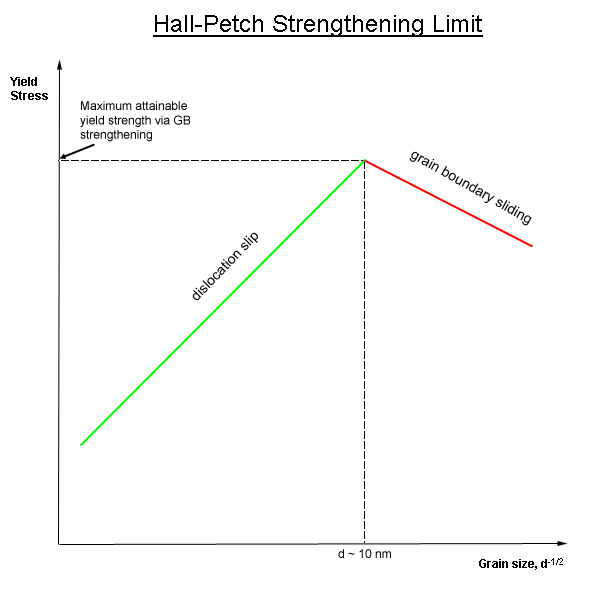
Grain Refinement
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. So, by changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.W.D. Callister. Fundamentals of Materials Science and Engineering, 2nd ed. Wiley & Sons. pp. 252. Theory In grain-boundary strengthening, the grain boundaries act as pinning points impeding further dislocation propagation. Since the lattice struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystalline Polycrystalline Amorphous
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bronze Bell With Visible Material Structure
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such as arsenic or silicon. These additions produce a range of alloys that may be harder than copper alone, or have other useful properties, such as strength, ductility, or machinability. The archaeological period in which bronze was the hardest metal in widespread use is known as the Bronze Age. The beginning of the Bronze Age in western Eurasia and India is conventionally dated to the mid-4th millennium BCE (~3500 BCE), and to the early 2nd millennium BCE in China; elsewhere it gradually spread across regions. The Bronze Age was followed by the Iron Age starting from about 1300 BCE and reaching most of Eurasia by about 500 BCE, although bronze continued to be much more widely used than it is in modern times. Because historical artworks we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ..., the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a big influence on hardness, density, Transparency and translucency, transparency and diffusion. In an ideal gas, the relative positions of the atoms or molecules are completely random. Amorphous materials, such as liquids and glasses, represent an intermediate case, having order over short distances (a few atomic or molecular spacings) but not over longer distances. Many materials, such as glass-ceramics and some polymers, can be prepared in such a way as to produce a mixture of crystalline and Amorphous solid, amorphous regions. In such cases, crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monocrystalline Silicon
Monocrystalline silicon, more often called single-crystal silicon, in short mono c-Si or mono-Si, is the base material for silicon-based discrete components and integrated circuits used in virtually all modern electronic equipment. Mono-Si also serves as a photovoltaic, light-absorbing material in the manufacture of solar cells. It consists of silicon in which the crystal lattice of the entire solid is continuous, unbroken to its edges, and free of any grain boundaries (i.e. a single crystal). Mono-Si can be prepared as an intrinsic semiconductor that consists only of exceedingly pure silicon, or it can be doped by the addition of other elements such as boron or phosphorus to make p-type or n-type silicon. Due to its semiconducting properties, single-crystal silicon is perhaps the most important technological material of the last few decades—the "silicon era", because its availability at an affordable cost has been essential for the development of the electronic devices on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Several key characteristics of a superalloy are excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic (FCC) austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys. Superalloy development has relied heavily on both chemical and process innovations. Superalloys develop high temperature strength through solid solution strengthening and precipitation strengthening from secondary phase precipitates such as gamma prime and carbides. Oxidation or corrosion resistance is provided by elements such as aluminium and chromium. Superalloys are often cast as a single crystal—while grain boundaries may provide strength at low temperatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single Crystal
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries.RIWD. "Reade Advanced Materials – Single Crystals". ''www.reade.com''. Retrieved 2021-02-28. The absence of the defects associated with grain boundaries can give monocrystals unique properties, particularly mechanical, optical and electrical, which can also be anisotropic, depending on the type of crystallographic structure. These properties, in addition to making some gems precious, are industrially used in technological applications, especially in optics and electronics. Because entropic effects favor the presence of some imperfections in the microstructure of solids, such as impurities, inhomogeneous strain and crystallographic defects such as dislocations, perfect single crystals of meaningful size are exceedingly rare in nature. The necess ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Solid
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal. Etymology The term comes from the Greek ''a'' ("without"), and ''morphé'' ("shape, form"). In some older articles and books, the term was used synonymously with glass. Today, "glassy solid" or "amorphous solid" is considered the overarching concept. Polymers are often amorphous. Structure Amorphous materials have an internal structure comprising interconnected structural blocks that can be similar to the basic structural units found in the corresponding crystalline phase of the same compound. Unlike crystalline materials, however, no long-range order exists. Localized order in amorphous materials can be categorized as short or medium range order. By convention, short range order extends only to the nearest neighbor shell, typically only 1-2 atomic spacings. Medium range order is then d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |