|
Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids. In inorganic chemistry and geology, carbonation is common. Metal hydroxides (MOH) and metal oxides (M'O) react with CO2 to give bicarbonates and carbonates: :MOH + CO2 → M(HCO3) :M'O + CO2 → M'CO3 In reinforced concrete, the chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate Calcium silicate is the chemical compound Ca2SiO4, also known as calcium orthosilicate and is sometimes formulated as 2CaO·SiO2. It is also referred to by the shortened trade name Cal-Sil or Calsil. It occurs naturally as the mineral larnite. ... in the concrete is known as Neutralization (chemistry), neutralisation. The similar reaction in which calcium hydroxide from cement reacts with carbon dioxide and for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
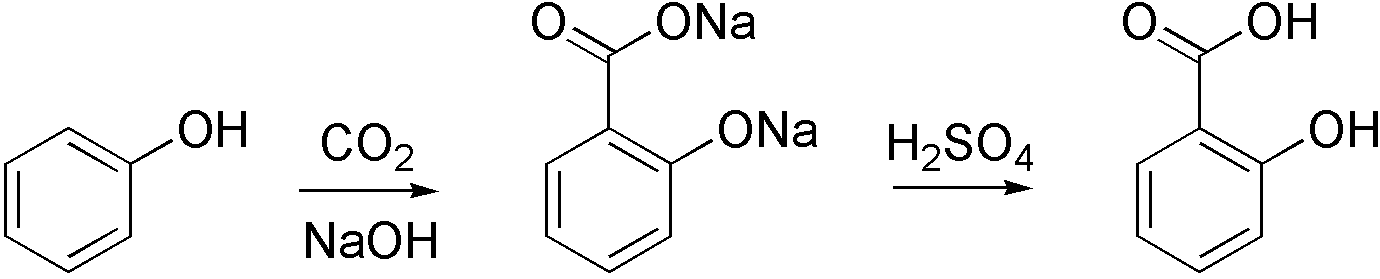
Carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reinforced Concrete
Reinforced concrete (RC), also called reinforced cement concrete (RCC) and ferroconcrete, is a composite material in which concrete's relatively low ultimate tensile strength, tensile strength and ductility are compensated for by the inclusion of reinforcement having higher tensile strength or ductility. The reinforcement is usually, though not necessarily, steel bars (rebar) and is usually embedded passively in the concrete before the concrete sets. However, Prestressed_concrete#Post-tensioned_concrete, post-tensioning is also employed as a technique to reinforce the concrete. In terms of volume used annually, it is one of the most common engineering materials. In corrosion engineering terms, when designed correctly, the alkalinity of the concrete protects the steel rebar from corrosion. Description Reinforcing schemes are generally designed to resist Tension (physics), tensile Stress (mechanics), stresses in particular regions of the concrete that might cause unacceptable frac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate: :Ca(OH)2CO2->CaCO3H_2O The process of forming a carbonate is sometimes referred to as "carbonation", although this term usually refers to the process of dissolving carbon dioxide in water. Concrete Carbonatation is a slow process that occurs in concrete where lime (CaO, or Ca(OH)2( aq)) in the cement reacts with carbon dioxide (CO2) from the air and forms calcium carbonate. The water in the pores of Portland cement concrete is normally alkaline with a pH in the range of 12.5 to 13.5. This highly alkaline environment is one in which the steel rebar is passivated and is protected from corrosion. According to the Pourbaix diagram for iron, the metal is passive when the pH is above 9.5.{{cite web , url=http://www.corrosion-doctors.org/Thermo/ironE-pH.htm , title=Pourbaix diagram of iron , publisher=Corrosion-doctors.org , date= , accessdate= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound is ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole Fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This expression is given below: :x_i = \frac The sum of all the mole fractions is equal to 1: :\sum_^ n_i = n_\mathrm ; \ \sum_^ x_i = 1. The same concept expressed with a denominator of 100 is the mole percent, molar percentage or molar proportion (mol%). The mole fraction is also called the amount fraction. It is identical to the number fraction, which is defined as the number of molecules of a constituent ''Ni'' divided by the total number of all molecules ''N''tot. The mole fraction is sometimes denoted by the lowercase Greek letter ( chi) instead of a Roman ''x''. For mixtures of gases, IUPAC recommends the letter ''y''. The National Institute of Standards and Technology of the United States prefers the term amount-of-substance fraction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry's Law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulated by the English chemist William Henry, who studied the topic in the early 19th century. An example where Henry's law is at play is in the depth-dependent dissolution of oxygen and nitrogen in the blood of underwater divers that changes during decompression, leading to decompression sickness. An everyday example is given by one's experience with carbonated soft drinks, which contain dissolved carbon dioxide. Before opening, the gas above the drink in its container is almost pure carbon dioxide, at a pressure higher than atmospheric pressure. After the bottle is opened, this gas escapes, moving the partial pressure of carbon dioxide above the liquid to be much lower, resulting in degassing as the dissolved carbon dioxide comes out of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants. Meaning of "neutralization" In the context of a chemical reaction the term neutralization is used for a reaction between an acid and a base or alkali. Historically, this reaction was represented as :acid + base (alkali) → salt + water For example: :HCl + NaOH → NaCl + H2O The statement is still valid as long as it is understood that in an aqueous solution the substances involved are subject to dissociation, which changes the ionization state of the substances. The arrow sign, →, is used because the reaction is complete, that is, neutralization is a quantitative reaction. A more general definition is bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Silicate
Calcium silicate is the chemical compound Ca2SiO4, also known as calcium orthosilicate and is sometimes formulated as 2CaO·SiO2. It is also referred to by the shortened trade name Cal-Sil or Calsil. It occurs naturally as the mineral larnite. Properties Calcium silicate is a white free-flowing powder. It can be derived from naturally occurring limestone and diatomaceous earth, a siliceous sedimentary rock. It is one of a group of compounds that can be produced by reacting calcium oxide and silica in various ratios e.g. 3CaO·SiO2, alite (Ca3SiO5); 2CaO·SiO2, (Ca2SiO4); 3CaO·2SiO2, (Ca3SiO7); and CaO·SiO2, wollastonite (CaSiO3). It has a low bulk density and high physical water absorption. Use Calcium silicate is used as an anticaking agent in food preparation, including table salt and as an |