Zinc Oxide on:
[Wikipedia]
[Google]
[Amazon]
Zinc oxide is an
 ZnO also forms cement-like material when treated with phosphoric acid; related materials are used in dentistry. A major component of zinc phosphate cement produced by this reaction is hopeite, Zn3(PO4)2·4H2O.
ZnO decomposes into zinc vapor and oxygen at around 1975 °C with a standard oxygen pressure. In a
ZnO also forms cement-like material when treated with phosphoric acid; related materials are used in dentistry. A major component of zinc phosphate cement produced by this reaction is hopeite, Zn3(PO4)2·4H2O.
ZnO decomposes into zinc vapor and oxygen at around 1975 °C with a standard oxygen pressure. In a
Applications of ZnO.
Access date January 25, 2009.

 ZnO has wide direct band gap (3.37 eV or 375 nm at room temperature). Therefore, its most common potential applications are in laser diodes and light emitting diodes (LEDs). Moreover, ultrafast nonlinearities and photoconductive functions have been reported in ZnO. Some optoelectronic applications of ZnO overlap with that of GaN, which has a similar band gap (~3.4 eV at room temperature). Compared to GaN, ZnO has a larger exciton binding energy (~60 meV, 2.4 times of the room-temperature thermal energy), which results in bright room-temperature emission from ZnO. ZnO can be combined with GaN for LED-applications. For instance, a transparent conducting oxide layer and ZnO nanostructures provide better light outcoupling. Other properties of ZnO favorable for electronic applications include its stability to high-energy radiation and its ability to be patterned by wet chemical etching. Radiation resistance makes ZnO a suitable candidate for space applications. ZnO is the most promising candidate in the field of random lasers to produce an electronically pumped UV laser source.
The pointed tips of ZnO nanorods result in a strong enhancement of an electric field. Therefore, they can be used as field emitters.
Aluminium-doped ZnO layers are used as transparent
ZnO has wide direct band gap (3.37 eV or 375 nm at room temperature). Therefore, its most common potential applications are in laser diodes and light emitting diodes (LEDs). Moreover, ultrafast nonlinearities and photoconductive functions have been reported in ZnO. Some optoelectronic applications of ZnO overlap with that of GaN, which has a similar band gap (~3.4 eV at room temperature). Compared to GaN, ZnO has a larger exciton binding energy (~60 meV, 2.4 times of the room-temperature thermal energy), which results in bright room-temperature emission from ZnO. ZnO can be combined with GaN for LED-applications. For instance, a transparent conducting oxide layer and ZnO nanostructures provide better light outcoupling. Other properties of ZnO favorable for electronic applications include its stability to high-energy radiation and its ability to be patterned by wet chemical etching. Radiation resistance makes ZnO a suitable candidate for space applications. ZnO is the most promising candidate in the field of random lasers to produce an electronically pumped UV laser source.
The pointed tips of ZnO nanorods result in a strong enhancement of an electric field. Therefore, they can be used as field emitters.
Aluminium-doped ZnO layers are used as transparent
 In form of a thin film ZnO has been demonstrated in miniaturised high frequency thin film resonators, sensors and filters.
In form of a thin film ZnO has been demonstrated in miniaturised high frequency thin film resonators, sensors and filters.
Zincite properties
*
Zinc white pigment
at ColourLex {{Authority control
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemis ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically.
ZnO is a wide-band gap semiconductor of the II-VI semiconductor group. The native doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility
In solid-state physics, the electron mobility characterises how quickly an electron can move through a metal or semiconductor when pulled by an electric field. There is an analogous quantity for holes, called hole mobility. The term carrier mobil ...
, wide band gap, and strong room-temperature luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crys ...
. Those properties make ZnO valuable for a variety of emerging applications: transparent electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
s in liquid crystal display
A liquid-crystal display (LCD) is a flat-panel display or other electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers. Liquid crystals do not emit light directly but ...
s, energy-saving or heat-protecting windows, and electronics as thin-film transistor
upright=1.4, gate (G), body (B), source (S) and drain (D) terminals. The gate is separated from the body by an insulating layer (pink).
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch ...
s and light-emitting diode
A light-emitting diode (LED) is a semiconductor Electronics, device that Light#Light sources, emits light when Electric current, current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy i ...
s.
Chemical properties
Pure ZnO is a white powder, but in nature it occurs as the rare mineral zincite, which usually contains manganese and other impurities that confer a yellow to red color. Crystalline zinc oxide is thermochromic, changing from white to yellow when heated in air and reverting to white on cooling. This color change is caused by a small loss of oxygen to the environment at high temperatures to form the non-stoichiometric Zn1+xO, where at 800 °C, x = 0.00007. Zinc oxide is anamphoteric oxide
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
One type of amphoteric species are amphiprot ...
. It is nearly insoluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in water, but it will dissolve in most acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
s, such as hydrochloric acid:
:ZnO + 2 HCl → ZnCl2 + H2O
Solid zinc oxide will also dissolve in alkalis to give soluble zincates:
:ZnO + 2 NaOH + H2O → Na2 n(OH)4
ZnO reacts slowly with fatty acids in oils to produce the corresponding carboxylates, such as oleate or stearate. When mixed with a strong aqueous solution of zinc chloride
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic ...
, ZnO forms cement-like products best described as zinc hydroxy chlorides. This cement was used in dentistry.
 ZnO also forms cement-like material when treated with phosphoric acid; related materials are used in dentistry. A major component of zinc phosphate cement produced by this reaction is hopeite, Zn3(PO4)2·4H2O.
ZnO decomposes into zinc vapor and oxygen at around 1975 °C with a standard oxygen pressure. In a
ZnO also forms cement-like material when treated with phosphoric acid; related materials are used in dentistry. A major component of zinc phosphate cement produced by this reaction is hopeite, Zn3(PO4)2·4H2O.
ZnO decomposes into zinc vapor and oxygen at around 1975 °C with a standard oxygen pressure. In a carbothermic reaction
Carbothermic reactions involve the reduction of substances, often metal oxides (O^2-), using carbon as the reducing agent. These chemical reactions are usually conducted at temperatures of several hundred degrees Celsius. Such processes are applie ...
, heating with carbon converts the oxide into zinc vapor at a much lower temperature (around 950 °C).
:ZnO + C → Zn(Vapor) + CO
Physical properties

Structure
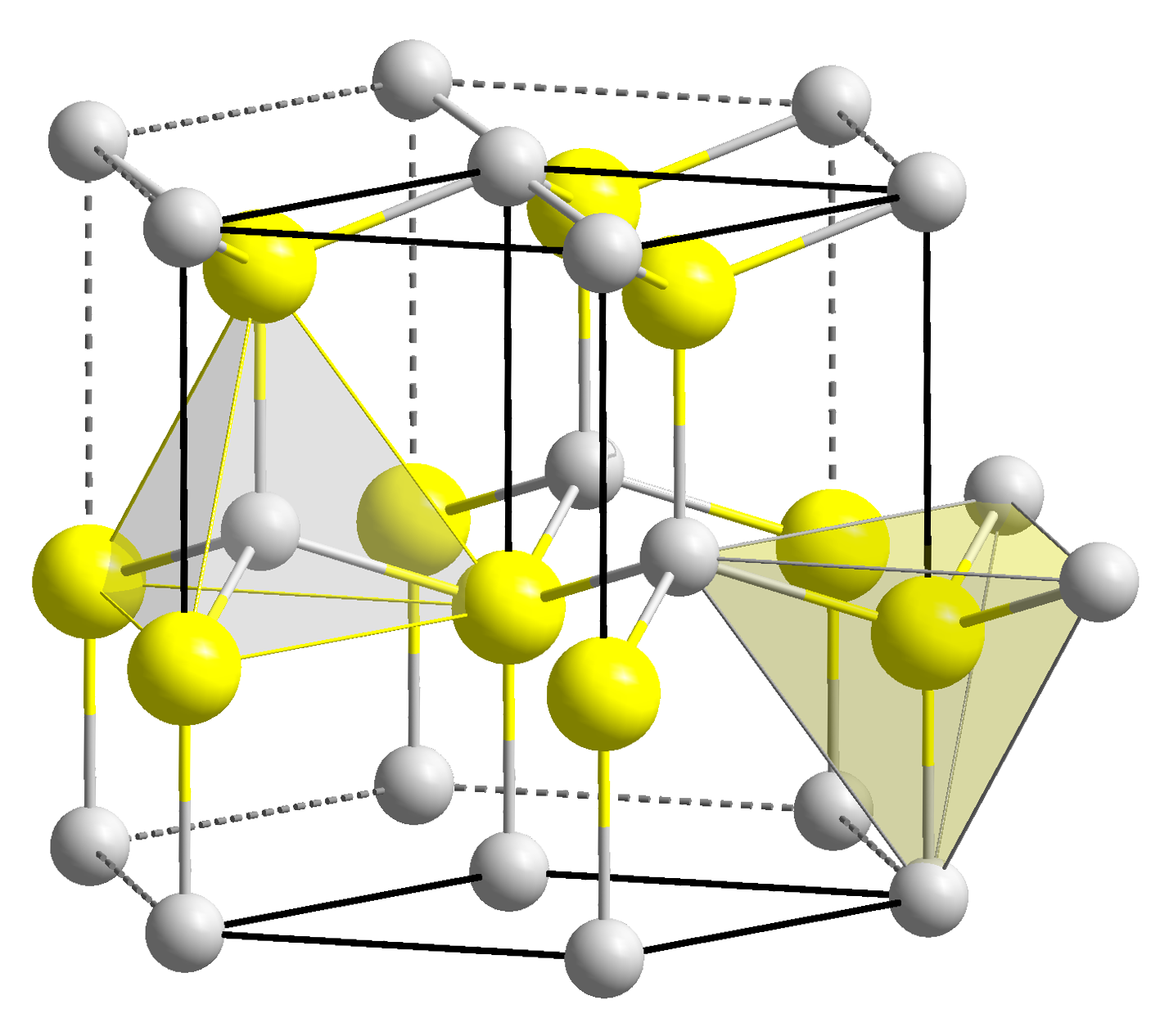
Zinc oxide crystallizes in two main forms, hexagonal wurtzite and cubic zincblende. The wurtzite structure is most stable at ambient conditions and thus most common. The zincblende form can be stabilized by growing ZnO on substrates with cubic lattice structure. In both cases, the zinc and oxide centers are tetrahedral, the most characteristic geometry for Zn(II). ZnO converts to therocksalt
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, p ...
motif at relatively high pressures about 10 GPa. The many remarkable medical properties of creams containing ZnO can be explained by its elastic softness, which is characteristic of tetrahedral coordinated binary compounds close to the transition to octahedral structures.
Hexagonal and zincblende polymorphs have no inversion symmetry (reflection of a crystal relative to any given point does not transform it into itself). This and other lattice symmetry properties result in piezoelectricity
Piezoelectricity (, ) is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word ' ...
of the hexagonal and zincblende ZnO, and pyroelectricity of hexagonal ZnO.
The hexagonal structure has a point group 6 mm ( Hermann–Mauguin notation) or C6v ( Schoenflies notation), and the space group is P63mc or C6v4. The lattice constants are ''a'' = 3.25 Å and ''c'' = 5.2 Å; their ratio ''c/a'' ~ 1.60 is close to the ideal value for hexagonal cell ''c/a'' = 1.633. As in most group II-VI materials, the bonding in ZnO is largely ionic (Zn2+O2−) with the corresponding radii of 0.074 nm for Zn2+ and 0.140 nm for O2−. This property accounts for the preferential formation of wurtzite rather than zinc blende structure, as well as the strong piezoelectricity
Piezoelectricity (, ) is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word ' ...
of ZnO. Because of the polar Zn−O bonds, zinc and oxygen planes are electrically charged. To maintain electrical neutrality, those planes reconstruct at atomic level in most relative materials, but not in ZnO – its surfaces are atomically flat, stable and exhibit no reconstruction. However, studies using wurtzoid structures explained the origin of surface flatness and the absence of reconstruction at ZnO wurtzite surfaces in addition to the origin of charges on ZnO planes.
Mechanical properties
ZnO is a relatively soft material with approximate hardness of 4.5 on theMohs scale
The Mohs scale of mineral hardness () is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of various minerals through the ability of harder material to scratch softer material.
The scale was introduced in 1812 by ...
. Its elastic constants are smaller than those of relevant III-V semiconductors, such as GaN. The high heat capacity and heat conductivity, low thermal expansion and high melting temperature of ZnO are beneficial for ceramics. The E2 optical phonon in ZnO exhibits an unusually long lifetime of 133 ps at 10 K.
Among the tetrahedrally bonded semiconductors, it has been stated that ZnO has the highest piezoelectric tensor, or at least one comparable to that of GaN and AlN
Aluminium nitride ( Al N) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potenti ...
. This property makes it a technologically important material for many piezoelectrical applications, which require a large electromechanical coupling. Therefore, ZnO in the form of thin film
A thin film is a layer of material ranging from fractions of a nanometer ( monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films (a process referred to as deposition) is a fundamental step in many ...
has been one of the most studied resonator materials for thin-film bulk acoustic resonator A thin-film bulk acoustic resonator (FBAR or TFBAR) is a device consisting of a piezoelectric material manufactured by thin film methods between two conductive – typically metallic – electrodes and acoustically isolated from the surrounding medi ...
s.
Electrical and optical properties
ZnO has a relatively largedirect
Direct may refer to:
Mathematics
* Directed set, in order theory
* Direct limit of (pre), sheaves
* Direct sum of modules, a construction in abstract algebra which combines several vector spaces
Computing
* Direct access (disambiguation), ...
band gap of ~3.3 eV at room temperature. Advantages associated with a large band gap include higher breakdown voltages
The breakdown voltage of an insulator is the minimum voltage that causes a portion of an insulator to experience electrical breakdown and become electrically conductive.
For diodes, the breakdown voltage is the minimum reverse voltage that ma ...
, ability to sustain large electric fields, lower electronic noise, and high-temperature and high-power operation. The band gap of ZnO can further be tuned to ~3–4 eV by its alloying with magnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2� ...
or cadmium oxide.
Most ZnO has ''n''-type character, even in the absence of intentional doping. Nonstoichiometry
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); mo ...
is typically the origin of n-type character, but the subject remains controversial. An alternative explanation has been proposed, based on theoretical calculations, that unintentional substitutional hydrogen impurities are responsible. Controllable n-type doping is easily achieved by substituting Zn with group-III elements such as Al, Ga, In or by substituting oxygen with group-VII elements chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
or iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
.
Reliable p-type doping of ZnO remains difficult. This problem originates from low solubility of p-type dopants and their compensation by abundant n-type impurities. This problem is observed with GaN and ZnSe
Zinc selenide (ZnSe) is a light-yellow, solid compound comprising zinc (Zn) and selenium (Se). It is an intrinsic semiconductor with a band gap of about 2.70 eV at . ZnSe rarely occurs in nature, and is found in the mineral that was named af ...
. Measurement of p-type in "intrinsically" n-type material is complicated by the inhomogeneity of samples.
Current limitations to p-doping limit electronic and optoelectronic applications of ZnO, which usually require junctions of n-type and p-type material. Known p-type dopants include group-I elements Li, Na, K; group-V elements N, P and As; as well as copper and silver. However, many of these form deep acceptors and do not produce significant p-type conduction at room temperature.
Electron mobility
In solid-state physics, the electron mobility characterises how quickly an electron can move through a metal or semiconductor when pulled by an electric field. There is an analogous quantity for holes, called hole mobility. The term carrier mobil ...
of ZnO strongly varies with temperature and has a maximum of ~2000 cm2/(V·s) at 80 K. Data on hole mobility are scarce with values in the range 5–30 cm2/(V·s).
ZnO discs, acting as a varistor
A varistor is an electronic component with an electrical resistance that varies with the applied voltage. Also known as a voltage-dependent resistor (VDR), it has a nonlinear, non- ohmic current–voltage characteristic that is similar to that ...
, are the active material in most surge arrester
A 'surge protector'' (or spike suppressor, surge suppressor, surge diverter, surge protection device (SPD) or transient voltage surge suppressor (TVSS) is an appliance or device intended to protect electrical devices from voltage spikes in alt ...
s.
Zinc oxide is noted for its strongly nonlinear optical properties, especially in bulk. The nonlinearity of ZnO nanoparticles can be fine-tuned according to their size.
Production
For industrial use, ZnO is produced at levels of 105 tons per year by three main processes:Indirect process
In the indirect or French process, metallic zinc is melted in a graphite crucible and vaporized at temperatures above 907 °C (typically around 1000 °C). Zinc vapor reacts with the oxygen in the air to give ZnO, accompanied by a drop in its temperature and bright luminescence. Zinc oxide particles are transported into a cooling duct and collected in a bag house. This indirect method was popularized by LeClaire (France) in 1844 and therefore is commonly known as the French process. Its product normally consists of agglomerated zinc oxide particles with an average size of 0.1 to a few micrometers. By weight, most of the world's zinc oxide is manufactured via French process.Direct process
The direct or American process starts with diverse contaminated zinc composites, such as zinc ores or smelter by-products. The zinc precursors are reduced ( carbothermal reduction) by heating with a source of carbon such asanthracite
Anthracite, also known as hard coal, and black coal, is a hard, compact variety of coal that has a submetallic luster. It has the highest carbon content, the fewest impurities, and the highest energy density of all types of coal and is the hig ...
to produce zinc vapor, which is then oxidized as in the indirect process. Because of the lower purity of the source material, the final product is also of lower quality in the direct process as compared to the indirect one.
Wet chemical process
A small amount of industrial production involves wet chemical processes, which start with aqueous solutions of zinc salts, from which zinc carbonate or zinc hydroxide is precipitated. The solid precipitate is then calcined at temperatures around 800 °C.Laboratory synthesis
Numerous specialised methods exist for producing ZnO for scientific studies and niche applications. These methods can be classified by the resulting ZnO form (bulk, thin film, nanowire), temperature ("low", that is close to room temperature or "high", that is T ~ 1000 °C), process type (vapor deposition or growth from solution) and other parameters. Large single crystals (many cubic centimeters) can be grown by the gas transport (vapor-phase deposition),hydrothermal synthesis
Hydrothermal synthesis includes the various techniques of crystallizing substances from high-temperature aqueous solutions at high vapor pressures; also termed "hydrothermal method". The term " hydrothermal" is of geologic origin. Geochemists and ...
, or melt growth. However, because of the high vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed pha ...
of ZnO, growth from the melt is problematic. Growth by gas transport is difficult to control, leaving the hydrothermal method as a preference. Thin films can be produced by chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
, metalorganic vapour phase epitaxy
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. ...
, electrodeposition, pulsed laser deposition, sputtering, sol–gel synthesis, atomic layer deposition
Atomic layer deposition (ALD) is a thin-film deposition technique based on the sequential use of a gas-phase chemical process; it is a subclass of chemical vapour deposition. The majority of ALD reactions use two chemicals called precursors (a ...
, spray pyrolysis, etc.
Ordinary white powdered zinc oxide can be produced in the laboratory by electrolyzing a solution of sodium bicarbonate with a zinc anode. Zinc hydroxide and hydrogen gas are produced. The zinc hydroxide upon heating decomposes to zinc oxide:
: Zn + 2 H2O → Zn(OH)2 + H2
: Zn(OH)2 → ZnO + H2O
ZnO nanostructures
Nanostructures of ZnO can be synthesized into a variety of morphologies including nanowires, nanorods, tetrapods, nanobelts, nanoflowers, nanoparticles etc. Nanostructures can be obtained with most above-mentioned techniques, at certain conditions, and also with thevapor–liquid–solid method
The vapor–liquid–solid method (VLS) is a mechanism for the growth of one-dimensional structures, such as nanowires, from chemical vapor deposition. The growth of a crystal through direct adsorption of a gas phase on to a solid surface is gene ...
. The synthesis is typically carried out at temperatures of about 90 °C, in an equimolar aqueous solution of zinc nitrate and hexamine, the latter providing the basic environment. Certain additives, such as polyethylene glycol or polyethylenimine, can improve the aspect ratio of the ZnO nanowires. Doping of the ZnO nanowires has been achieved by adding other metal nitrates to the growth solution. The morphology of the resulting nanostructures can be tuned by changing the parameters relating to the precursor composition (such as the zinc concentration and pH) or to the thermal treatment (such as the temperature and heating rate).
Aligned ZnO nanowires on pre-seeded silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
, glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
, and gallium nitride substrates have been grown using aqueous zinc salts such as zinc nitrate and zinc acetate in basic environments. Pre-seeding substrates with ZnO creates sites for homogeneous nucleation of ZnO crystal during the synthesis. Common pre-seeding methods include in-situ thermal decomposition of zinc acetate crystallites, spincoating of ZnO nanoparticles and the use of physical vapor deposition methods to deposit ZnO thin films. Pre-seeding can be performed in conjunction with top down patterning methods such as electron beam lithography and nanosphere lithography to designate nucleation sites prior to growth. Aligned ZnO nanowires can be used in dye-sensitized solar cell
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a '' photoelec ...
s and field emission devices.
History
Zinc compounds were probably used by early humans, in processed and unprocessed forms, as a paint or medicinal ointment, but their composition is uncertain. The use of ''pushpanjan'', probably zinc oxide, as a salve for eyes and open wounds, is mentioned in the Indian medical text theCharaka Samhita
The ''Charaka Samhita'' (, “Compendium of '' Charaka''”) is a Sanskrit text on Ayurveda (Indian traditional medicine). Along with the '' Sushruta Samhita'', it is one of the two foundational texts of this field that have survived from anci ...
, thought to date from 500 BC or before. Zinc oxide ointment is also mentioned by the Greek physician Dioscorides
Pedanius Dioscorides ( grc-gre, Πεδάνιος Διοσκουρίδης, ; 40–90 AD), “the father of pharmacognosy”, was a Greek physician, pharmacologist, botanist, and author of '' De materia medica'' (, On Medical Material) —a 5-vo ...
(1st century AD). Galen
Aelius Galenus or Claudius Galenus ( el, Κλαύδιος Γαληνός; September 129 – c. AD 216), often Anglicized as Galen () or Galen of Pergamon, was a Greek physician, surgeon and philosopher in the Roman Empire. Considered to be o ...
suggested treating ulcerating cancers with zinc oxide, as did Avicenna
Ibn Sina ( fa, ابن سینا; 980 – June 1037 CE), commonly known in the West as Avicenna (), was a Persian polymath who is regarded as one of the most significant physicians, astronomers, philosophers, and writers of the Islamic ...
in his '' The Canon of Medicine''. It is used as an ingredient in products such as baby powder
Baby powder is an astringent powder used for preventing diaper rash and for cosmetic uses. It may be composed of talc (in which case it is also called talcum powder) or corn starch. It may also contain additional ingredients like fragrances. ...
and creams against diaper rash
Irritant diaper dermatitis (IDD, also called a diaper/nappy rash) is a generic term applied to skin rash in the diaper nappy area that are caused by various skin disorders and/or irritants.
Generic irritant diaper/nappy dermatitis is characteriz ...
es, calamine
Calamine, also known as calamine lotion, is a medication used to treat mild itchiness. This includes from sunburn, insect bites, poison ivy, poison oak, and other mild skin conditions. It may also help dry out skin irritation. It is applied ...
cream, anti-dandruff
Dandruff is a skin condition that mainly affects the scalp. Symptoms include flaking and sometimes mild itchiness. It can result in social or self-esteem problems. A more severe form of the condition, which includes inflammation of the skin ...
shampoo
Shampoo () is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in solid bar format. Shampoo is used by applying it to wet hair, massaging the product into the ...
s, and antiseptic
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
ointments.
The Romans produced considerable quantities of brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other wi ...
(an alloy of zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
and copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
) as early as 200 BC by a cementation process where copper was reacted with zinc oxide. The zinc oxide is thought to have been produced by heating zinc ore in a shaft furnace. This liberated metallic zinc as a vapor, which then ascended the flue and condensed as the oxide. This process was described by Dioscorides
Pedanius Dioscorides ( grc-gre, Πεδάνιος Διοσκουρίδης, ; 40–90 AD), “the father of pharmacognosy”, was a Greek physician, pharmacologist, botanist, and author of '' De materia medica'' (, On Medical Material) —a 5-vo ...
in the 1st century AD. Zinc oxide has also been recovered from zinc mines at Zawar in India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area, the List of countries and dependencies by population, second-most populous ...
, dating from the second half of the first millennium BC.
From the 12th to the 16th century zinc and zinc oxide were recognized and produced in India using a primitive form of the direct synthesis process. From India, zinc manufacture moved to China in the 17th century. In 1743, the first European zinc smelter was established in Bristol
Bristol () is a City status in the United Kingdom, city, Ceremonial counties of England, ceremonial county and unitary authority in England. Situated on the River Avon, Bristol, River Avon, it is bordered by the ceremonial counties of Glouces ...
, United Kingdom. Around 1782 Louis-Bernard Guyton de Morveau proposed replacing lead white with zinc oxide.
The main usage of zinc oxide (zinc white) was in paints and as an additive to ointments. Zinc white was accepted as a pigment in oil paintings by 1834 but it did not mix well with oil. This problem was solved by optimizing the synthesis of ZnO. In 1845, LeClaire in Paris was producing the oil paint on a large scale, and by 1850, zinc white was being manufactured throughout Europe. The success of zinc white paint was due to its advantages over the traditional white lead: zinc white is essentially permanent in sunlight, it is not blackened by sulfur-bearing air, it is non-toxic and more economical. Because zinc white is so "clean" it is valuable for making tints with other colors, but it makes a rather brittle dry film when unmixed with other colors. For example, during the late 1890s and early 1900s, some artists used zinc white as a ground for their oil paintings. All those paintings developed cracks over the years.
In recent times, most zinc oxide was used in the rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
industry to resist corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
. In the 1970s, the second largest application of ZnO was photocopying
A photocopier (also called copier or copy machine, and formerly Xerox machine, the generic trademark) is a machine that makes copies of documents and other visual images onto paper or plastic film quickly and cheaply. Most modern photocopier ...
. High-quality ZnO produced by the "French process" was added to photocopying paper as a filler. This application was soon displaced by titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
.
Applications
The applications of zinc oxide powder are numerous, and the principal ones are summarized below. Most applications exploit the reactivity of the oxide as a precursor to other zinc compounds. For material science applications, zinc oxide has highrefractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, ...
, high thermal conductivity, binding, antibacterial and UV-protection properties. Consequently, it is added into materials and products including plastics, ceramics, glass, cement, rubber, lubricants, paints, ointments, adhesive, sealants, concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most wid ...
manufacturing, pigments, foods, batteries, ferrites, fire retardants, etc.
Rubber manufacture
Between 50% and 60% of ZnO use is in the rubber industry. Zinc oxide along withstearic acid
Stearic acid ( , ) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a waxy solid and its chemical formula is C17H35CO2H. Its name comes from the Greek word στέαρ "''stéar''", which means ta ...
is used in the vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
of rubber ZnO additives also protect rubber from fungi (see medical applications) and UV light.
Ceramic industry
Ceramic industry consumes a significant amount of zinc oxide, in particular in ceramic glaze and frit compositions. The relatively high heat capacity, thermal conductivity and high temperature stability of ZnO coupled with a comparatively low coefficient of expansion are desirable properties in the production of ceramics. ZnO affects the melting point and optical properties of the glazes, enamels, and ceramic formulations. Zinc oxide as a low expansion, secondary flux improves the elasticity of glazes by reducing the change in viscosity as a function of temperature and helps prevent crazing and shivering. By substituting ZnO for BaO and PbO, the heat capacity is decreased and the thermal conductivity is increased. Zinc in small amounts improves the development of glossy and brilliant surfaces. However, in moderate to high amounts, it produces matte and crystalline surfaces. With regard to color, zinc has a complicated influence.Medicine
Zinc oxide as a mixture with about 0.5%iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally a ...
(Fe2O3) is called calamine
Calamine, also known as calamine lotion, is a medication used to treat mild itchiness. This includes from sunburn, insect bites, poison ivy, poison oak, and other mild skin conditions. It may also help dry out skin irritation. It is applied ...
and is used in calamine lotion. Two minerals, zincite and hemimorphite, have been historically called calamine
Calamine, also known as calamine lotion, is a medication used to treat mild itchiness. This includes from sunburn, insect bites, poison ivy, poison oak, and other mild skin conditions. It may also help dry out skin irritation. It is applied ...
. When mixed with eugenol
Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, bas ...
, a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
, zinc oxide eugenol
Zinc oxide eugenol (ZOE) is a material created by the combination of zinc oxide and eugenol contained in oil of cloves. An acid-base reaction takes place with the formation of zinc eugenolate chelate. The reaction is catalysed by water and is a ...
is formed, which has applications as a restorative and prosthodontic in dentistry
Dentistry, also known as dental medicine and oral medicine, is the branch of medicine focused on the teeth, gums, and mouth. It consists of the study, diagnosis, prevention, management, and treatment of diseases, disorders, and conditions of ...
.
Reflecting the basic properties of ZnO, fine particles of the oxide have deodorizing and antibacterial properties and for that reason are added into materials including cotton fabric, rubber, oral care products, and food packaging. Enhanced antibacterial action of fine particles compared to bulk material is not exclusive to ZnO and is observed for other materials, such as silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
. This property results from the increased surface area of the fine particles.
Zinc oxide is used in mouthwash products and toothpaste
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, ...
s as an anti-bacterial agent proposed to prevent plaque and tartar
Tartar may refer to:
Places
* Tartar (river), a river in Azerbaijan
* Tartar, Switzerland, a village in the Grisons
* Tərtər, capital of Tartar District, Azerbaijan
* Tartar District, Azerbaijan
* Tartar Island, South Shetland Islands, A ...
formation, and to control bad breath by reducing the volatile gases and volatile sulfur compounds (VSC) in the mouth. Along with zinc oxide or zinc salts, these products also commonly contain other active ingredients, such as cetylpyridinium chloride, xylitol, hinokitiol, essential oils and plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae excl ...
extract
An extract is a substance made by extracting a part of a raw material, often by using a solvent such as ethanol, oil or water. Extracts may be sold as tinctures, absolutes or in powder form.
The aromatic principles of many spices, nuts ...
s.
Zinc oxide is widely used to treat a variety of skin conditions, including atopic dermatitis
Atopic dermatitis (AD), also known as atopic eczema, is a long-term type of inflammation of the skin ( dermatitis). It results in itchy, red, swollen, and cracked skin. Clear fluid may come from the affected areas, which often thickens over tim ...
, contact dermatitis
Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes are ...
, itching due to eczema
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved c ...
, diaper rash
Irritant diaper dermatitis (IDD, also called a diaper/nappy rash) is a generic term applied to skin rash in the diaper nappy area that are caused by various skin disorders and/or irritants.
Generic irritant diaper/nappy dermatitis is characteriz ...
and acne
Acne, also known as ''acne vulgaris'', is a long-term skin condition that occurs when dead skin cells and oil from the skin clog hair follicles. Typical features of the condition include blackheads or whiteheads, pimples, oily skin, and ...
. Zinc oxide is also often added into sunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunbur ...
s.
It is used in products such as baby powder
Baby powder is an astringent powder used for preventing diaper rash and for cosmetic uses. It may be composed of talc (in which case it is also called talcum powder) or corn starch. It may also contain additional ingredients like fragrances. ...
and barrier creams to treat diaper rash
Irritant diaper dermatitis (IDD, also called a diaper/nappy rash) is a generic term applied to skin rash in the diaper nappy area that are caused by various skin disorders and/or irritants.
Generic irritant diaper/nappy dermatitis is characteriz ...
es, calamine
Calamine, also known as calamine lotion, is a medication used to treat mild itchiness. This includes from sunburn, insect bites, poison ivy, poison oak, and other mild skin conditions. It may also help dry out skin irritation. It is applied ...
cream, anti-dandruff
Dandruff is a skin condition that mainly affects the scalp. Symptoms include flaking and sometimes mild itchiness. It can result in social or self-esteem problems. A more severe form of the condition, which includes inflammation of the skin ...
shampoo
Shampoo () is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in solid bar format. Shampoo is used by applying it to wet hair, massaging the product into the ...
s, and antiseptic
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
ointments. It is also a component in tape (called "zinc oxide tape") used by athletes as a bandage to prevent soft tissue damage during workouts.
Zinc oxide can be used in ointments, creams, and lotions to protect against sunburn
Sunburn is a form of radiation burn that affects living tissue, such as skin, that results from an overexposure to ultraviolet (UV) radiation, usually from the Sun. Common symptoms in humans and animals include: red or reddish skin that is h ...
and other damage to the skin caused by ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiatio ...
(see sunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunbur ...
). It is the broadest spectrum UVA and UVB absorber that is approved for use as a sunscreen by the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ...
(FDA), and is completely photostable. When used as an ingredient in sunscreen, zinc oxide blocks both UVA (320–400 nm) and UVB (280–320 nm) rays of ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiatio ...
. Zinc oxide and the other most common physical sunscreen, titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolu ...
, are considered to be nonirritating, nonallergenic, and non-comedogenic
The term acne cosmetica refers to acne caused by or aggravated by cosmetics. The mechanism is thought to be chemically induced plugging of the pilosebaceous orifice. This became a significant problem for dermatologists in the 1970s and 1980s, b ...
. Zinc from zinc oxide is, however, slightly absorbed into the skin.
Many sunscreens use nanoparticles of zinc oxide (along with nanoparticles of titanium dioxide) because such small particles do not scatter light and therefore do not appear white. The nanoparticles are not absorbed into the skin more than regular-sized zinc oxide particles are, and are only absorbed into the outermost layer of the skin but not into the body.
Zinc oxide nanoparticles can enhance the antibacterial activity of ciprofloxacin
Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections. This includes bone and joint infections, intra abdominal infections, certain types of infectious diarrhea, respiratory tract infections, skin i ...
. It has been shown that nano ZnO that has an average size between 20 nm and 45 nm can enhance the antibacterial activity of ciprofloxacin
Ciprofloxacin is a fluoroquinolone antibiotic used to treat a number of bacterial infections. This includes bone and joint infections, intra abdominal infections, certain types of infectious diarrhea, respiratory tract infections, skin i ...
against ''Staphylococcus aureus
''Staphylococcus aureus'' is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often posit ...
'' and ''Escherichia coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Esc ...
'' in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology a ...
. The enhancing effect of this nanomaterial is concentration dependent against all test strains. This effect may be due to two reasons. First, zinc oxide nanoparticles can interfere with NorA protein, which is developed for conferring resistance in bacteria and has pumping activity that mediates the effluxing of hydrophilic fluoroquinolones from a cell. Second, zinc oxide nanoparticles can interfere with Omf protein, which is responsible for the permeation of quinolone antibiotic
A quinolone antibiotic is a member of a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as wel ...
s into the cell.
Cigarette filters
Zinc oxide is a component ofcigarette filter
A cigarette filter, also known as a filter tip, is a component of a cigarette, along with cigarette paper, capsules and adhesives. Filters were introduced in the early 1950s.
Filters may be made from plastic cellulose acetate fiber, paper or ...
s. A filter consisting of charcoal impregnated with zinc oxide and iron oxide removes significant amounts of hydrogen cyanide ( HCN) and hydrogen sulfide ( H2S) from tobacco smoke without affecting its flavor.Ambica Dhatu Private LimitedApplications of ZnO.
Access date January 25, 2009.
Food additive
Zinc oxide is added to many food products, includingbreakfast cereal
Cereal, formally termed breakfast cereal (and further categorized as cold cereal or warm cereal), is a traditional breakfast food made from processed cereal grains. It is traditionally eaten as part of breakfast, or a snack food, primarily in We ...
s, as a source of zinc, a necessary nutrient
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excre ...
. (Zinc sulfate
Zinc sulfate is an inorganic compound. It is used as a dietary supplement to treat zinc deficiency and to prevent the condition in those at high risk. Side effects of excess supplementation may include abdominal pain, vomiting, headache, and ti ...
is also used for the same purpose.) Some prepackaged foods also include trace amounts of ZnO even if it is not intended as a nutrient.
Zinc oxide was linked to dioxin contamination in pork exports in the 2008 Chilean pork crisis. The contamination was found to be due to dioxin contaminated zinc oxide used in pig feed.
Pigment
Zinc oxide (zinc white) is used as a pigment inpaint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s and is more opaque than lithopone, but less opaque than titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolu ...
. It is also used in coatings for paper. Chinese white is a special grade of zinc white used in artists' pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s. The use of zinc white as a pigment in oil painting started in the middle of 18th century. It has partly replaced the poisonous lead white and was used by painters such as Böcklin, Van Gogh
Vincent Willem van Gogh (; 30 March 185329 July 1890) was a Dutch Post-Impressionist painter who posthumously became one of the most famous and influential figures in Western art history. In a decade, he created about 2,100 artworks, inc ...
, Manet
A wireless ad hoc network (WANET) or mobile ad hoc network (MANET) is a decentralized type of wireless network. The network is ad hoc because it does not rely on a pre-existing infrastructure, such as routers in wired networks or access points ...
, Munch and others. It is also a main ingredient of mineral makeup (CI 77947).
UV absorber
Micronized and nano-scale zinc oxide provides strong protection against UVA and UVB ultraviolet radiation, and are consequently used insunscreens
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn an ...
, and also in UV-blocking sunglasses
Sunglasses or sun glasses (informally called shades or sunnies; more names Sunglasses#Other names, below) are a form of Eye protection, protective eyewear designed primarily to prevent bright sunlight and high-energy visible light from damagin ...
for use in space and for protection when welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as b ...
, following research by scientists at Jet Propulsion Laboratory ( JPL).
Coatings
Paints containing zinc oxide powder have long been utilized as anticorrosive coatings for metals. They are especially effective for galvanized iron. Iron is difficult to protect because its reactivity with organic coatings leads to brittleness and lack of adhesion. Zinc oxide paints retain their flexibility and adherence on such surfaces for many years. ZnO highly n-type doped withaluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, gallium
Gallium is a chemical element with the Symbol (chemistry), symbol Ga and atomic number 31. Discovered by France, French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in boron group, group 13 of the periodic table and is similar to ...
, or indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
is transparent and conductive ( transparency ~90%, lowest resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
~10−4 Ω·cm). ZnO:Al coatings are used for energy-saving or heat-protecting windows. The coating lets the visible part of the spectrum in but either reflects the infrared (IR) radiation back into the room (energy saving) or does not let the IR radiation into the room (heat protection), depending on which side of the window has the coating.
Plastics, such as polyethylene naphthalate (PEN), can be protected by applying zinc oxide coating. The coating reduces the diffusion of oxygen through PEN. Zinc oxide layers can also be used on polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
in outdoor applications. The coating protects polycarbonate from solar radiation, and decreases its oxidation rate and photo-yellowing.
Corrosion prevention in nuclear reactors
Zinc oxide depleted in 64Zn (the zinc isotope withatomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&n ...
64) is used in corrosion prevention in nuclear pressurized water reactor
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan and Canada). In a PWR, the primary coolant (water) i ...
s. The depletion is necessary, because 64Zn is transformed into radioactive 65Zn under irradiation by the reactor neutrons.
Methane reforming
Zinc oxide (ZnO) is used as a pretreatment step to removehydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The under ...
(H2S) from natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
following hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
of any sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
compounds prior to a methane reformer A methane reformer is a device based on steam reforming, autothermal reforming or partial oxidation and is a type of chemical synthesis which can produce pure hydrogen gas from methane using a catalyst. There are multiple types of reformers in dev ...
, which can poison the catalyst. At temperatures between about , H2S is converted to water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
by the following reaction:
:H2S + ZnO → H2O + ZnS
The zinc sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various ...
(ZnS) is replaced with fresh zinc oxide when the zinc oxide has been consumed.
Potential applications
Electronics

 ZnO has wide direct band gap (3.37 eV or 375 nm at room temperature). Therefore, its most common potential applications are in laser diodes and light emitting diodes (LEDs). Moreover, ultrafast nonlinearities and photoconductive functions have been reported in ZnO. Some optoelectronic applications of ZnO overlap with that of GaN, which has a similar band gap (~3.4 eV at room temperature). Compared to GaN, ZnO has a larger exciton binding energy (~60 meV, 2.4 times of the room-temperature thermal energy), which results in bright room-temperature emission from ZnO. ZnO can be combined with GaN for LED-applications. For instance, a transparent conducting oxide layer and ZnO nanostructures provide better light outcoupling. Other properties of ZnO favorable for electronic applications include its stability to high-energy radiation and its ability to be patterned by wet chemical etching. Radiation resistance makes ZnO a suitable candidate for space applications. ZnO is the most promising candidate in the field of random lasers to produce an electronically pumped UV laser source.
The pointed tips of ZnO nanorods result in a strong enhancement of an electric field. Therefore, they can be used as field emitters.
Aluminium-doped ZnO layers are used as transparent
ZnO has wide direct band gap (3.37 eV or 375 nm at room temperature). Therefore, its most common potential applications are in laser diodes and light emitting diodes (LEDs). Moreover, ultrafast nonlinearities and photoconductive functions have been reported in ZnO. Some optoelectronic applications of ZnO overlap with that of GaN, which has a similar band gap (~3.4 eV at room temperature). Compared to GaN, ZnO has a larger exciton binding energy (~60 meV, 2.4 times of the room-temperature thermal energy), which results in bright room-temperature emission from ZnO. ZnO can be combined with GaN for LED-applications. For instance, a transparent conducting oxide layer and ZnO nanostructures provide better light outcoupling. Other properties of ZnO favorable for electronic applications include its stability to high-energy radiation and its ability to be patterned by wet chemical etching. Radiation resistance makes ZnO a suitable candidate for space applications. ZnO is the most promising candidate in the field of random lasers to produce an electronically pumped UV laser source.
The pointed tips of ZnO nanorods result in a strong enhancement of an electric field. Therefore, they can be used as field emitters.
Aluminium-doped ZnO layers are used as transparent electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
s. The components Zn and Al are much cheaper and less toxic compared to the generally used indium tin oxide Indium tin oxide (ITO) is a ternary composition of indium, tin and oxygen in varying proportions. Depending on the oxygen content, it can be described as either a ceramic or an alloy. Indium tin oxide is typically encountered as an oxygen-saturated ...
(ITO). One application which has begun to be commercially available is the use of ZnO as the front contact for solar cells or of liquid crystal display
A liquid-crystal display (LCD) is a flat-panel display or other electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers. Liquid crystals do not emit light directly but ...
s.
Transparent thin-film transistor
upright=1.4, gate (G), body (B), source (S) and drain (D) terminals. The gate is separated from the body by an insulating layer (pink).
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch ...
s (TTFT) can be produced with ZnO. As field-effect transistors, they do not need a p–n junction, thus avoiding the p-type doping problem of ZnO. Some of the field-effect transistors even use ZnO nanorods as conducting channels.
Zinc oxide nanorod sensor
Zinc oxide nanorod sensors are devices detecting changes inelectric current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The movi ...
passing through zinc oxide nanowires due to adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
of gas molecules. Selectivity to hydrogen gas was achieved by sputtering palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
clusters on the nanorod surface. The addition of palladium appears to be effective in the catalytic dissociation of hydrogen molecules into atomic hydrogen, increasing the sensitivity of the sensor device. The sensor detects hydrogen concentrations down to 10 parts per million at room temperature, whereas there is no response to oxygen. ZnO have been used as immobilization layers in immunosensors enabling the distribution of antibodies across the entire region probed by the measuring electric field applied to the microelectrodes.
Spintronics
ZnO has also been considered forspintronics
Spintronics (a portmanteau meaning spin transport electronics), also known as spin electronics, is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid- ...
applications: if doped with 1–10% of magnetic ions (Mn, Fe, Co, V, etc.), ZnO could become ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
, even at room temperature. Such room temperature ferromagnetism
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
in ZnO:Mn has been observed, but it is not clear yet whether it originates from the matrix itself or from secondary oxide phases.
Piezoelectricity
Thepiezoelectricity
Piezoelectricity (, ) is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word ' ...
in textile
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, different #Fabric, fabric types, etc. At f ...
fibers coated in ZnO have been shown capable of fabricating "self-powered nanosystems" with everyday mechanical stress from wind or body movements.
In 2008 the ''Center for Nanostructure Characterization'' at the Georgia Institute of Technology
The Georgia Institute of Technology, commonly referred to as Georgia Tech or, in the state of Georgia, as Tech or The Institute, is a public research university and institute of technology in Atlanta, Georgia. Established in 1885, it is part ...
reported producing an electricity generating device (called flexible charge pump generator) delivering alternating current by stretching and releasing zinc oxide nanowires. This mini-generator creates an oscillating voltage up to 45 millivolts, converting close to seven percent of the applied mechanical energy into electricity. Researchers used wires with lengths of 0.2–0.3 mm and diameters of three to five micrometers, but the device could be scaled down to smaller size.
 In form of a thin film ZnO has been demonstrated in miniaturised high frequency thin film resonators, sensors and filters.
In form of a thin film ZnO has been demonstrated in miniaturised high frequency thin film resonators, sensors and filters.
Li-ion battery and supercapacitors
ZnO is a promising anode material forlithium-ion battery
A lithium-ion or Li-ion battery is a type of rechargeable battery which uses the reversible reduction of lithium ions to store energy. It is the predominant battery type used in portable consumer electronics and electric vehicles. It also s ...
because it is cheap, biocompatible, and environmentally friendly. ZnO has a higher theoretical capacity (978 mAh g−1) than many other transition metal oxides such as CoO (715 mAh g−1), NiO (718 mAh g−1) and CuO (674 mAh g−1). ZnO is also used as an electrode in supercapacitors.
Safety
As afood additive
Food additives are substances added to food to preserve Taste, flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), sal ...
, zinc oxide is on the U.S. FDA's list of generally recognized as safe, or GRAS, substances.
Zinc oxide itself is non-toxic; it is hazardous, however, to inhale zinc oxide fumes, such as generated when zinc or zinc alloys are melted and oxidized at high temperature. This problem occurs while melting alloys containing brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other wi ...
because the melting point of brass is close to the boiling point of zinc. Exposure to zinc oxide in the air, which also occurs while welding galvanized (zinc plated) steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
, can result in a malady called metal fume fever. For this reason, typically galvanized steel is not welded, or the zinc is removed first.
In sunscreen formulations containing zinc oxide and other UV absorbers, zinc oxide was found to cause photodegradation of small-molecule UV absorbers, which exhibited toxicity in embryonic zebrafish assays.
See also
* Depleted zinc oxide *Zinc oxide nanoparticle
Zinc oxide nanoparticles are nanoparticles of zinc oxide (ZnO) that have diameters less than 100 nanometers. They have a large surface area relative to their size and high catalytic activity. The exact physical and chemical properties of zinc oxi ...
* Gallium(III) nitride
* List of inorganic pigments
The following list includes commercially or artistically important inorganic pigments of natural and synthetic origin..
Purple pigments
Aluminum pigments
* Ultramarine violet: (PV15) - a synthetic or naturally occurring sulfur containing silic ...
* Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
* Zinc oxide eugenol
Zinc oxide eugenol (ZOE) is a material created by the combination of zinc oxide and eugenol contained in oil of cloves. An acid-base reaction takes place with the formation of zinc eugenolate chelate. The reaction is catalysed by water and is a ...
* Zinc peroxide
Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc peroxide has also been used as an oxidant in explosives and pyrotechnic mixtures. Its properties have ...
* Zinc smelting
* Zinc–air battery
Zinc–air batteries (non-rechargeable), and zinc–air fuel cells (mechanically rechargeable) are metal–air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexp ...
* Zinc–zinc oxide cycle
* ZnO nanostructures Zinc oxide (ZnO) nanostructures are structures with at least one dimension on the nanometre scale, composed predominantly of zinc oxide. They may be combined with other composite substances to change the chemistry, structure or function of the nano ...
References
Cited sources
*Reviews
* * * * * * * * * *External links
Zincite properties
*
Zinc white pigment
at ColourLex {{Authority control
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
Inorganic pigments
II-VI semiconductors
Corrosion inhibitors
Ceramic materials
Sunscreening agents
Amphoteric compounds
Antipruritics
Oxides
Piezoelectric materials
Nonlinear optical materials
Wurtzite structure type