superbase on:
[Wikipedia]
[Google]
[Amazon]
A superbase is a compound that has a particularly high affinity for
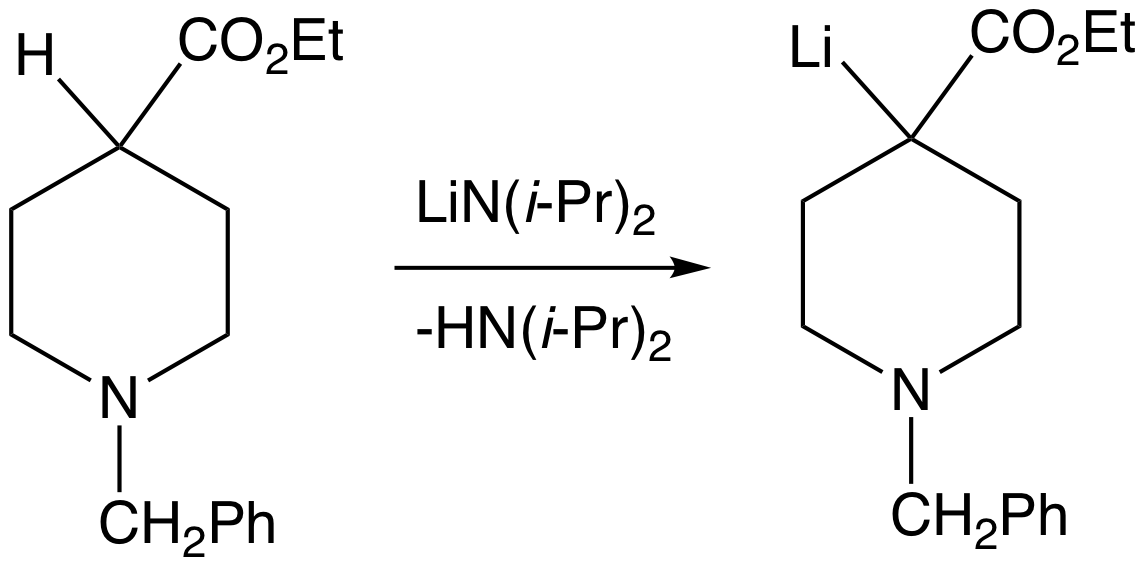 Organometallic compounds of electropositive metals are superbases, but they are generally strong nucleophiles. Examples include
Organometallic compounds of electropositive metals are superbases, but they are generally strong nucleophiles. Examples include
protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron m ...
. Superbases are of theoretical interest and potentially valuable in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. Superbases have been described and used since the 1850s.''Superbases for Organic Synthesis'' Ed. Ishikawa, T., John Wiley and Sons, Ltd.: West Sussex, UK. 2009.
Definitions
GenericallyIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
defines a superbase as a "compound having a very high basicity
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. R ...
, such as lithium diisopropylamide." Superbases are often defined in two broad categories, organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
and organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
.
Organic superbases are charge-neutral compounds with basicities greater than that of proton sponge
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula CH(NMe) (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional b ...
(pKBH+ = 18.6 in MeCN)." In a related definition: any species with a higher absolute proton affinity (APA = 245.3 kcal/mol) and intrinsic gas phase basicity (GB = 239 kcal/mol) than proton sponge. Common superbases of this variety feature amidine, guanidine, and phosphazene functional groups. Strong superbases can be designed by utilizing multiple intramolecular hydrogen bonds that stabilize the conjugate acid.
Organometallic superbases, sometimes called Lochmann–Schlosser superbases, result from the combination of alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
alkoxides
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, w ...
and organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
reagents. Caubère defines superbases as "bases resulting from a mixing of two (or more) bases leading to new basic species possessing inherent new properties. The term ''superbase'' does not mean a base is thermodynamically and/or kinetically stronger than another, instead it means that a basic reagent is created by combining the characteristics of several different bases."
Organic superbases
290 px, left, Protonation of Verkade base. Its conjugate acid has a pKa of 32.9 in acetonitrile. Organic superbases are mostly charge-neutral, nitrogen containing species, where nitrogen act as a proton acceptor. These include the phosphazenes, phosphanes, amidines, and guanidines. Other organic compounds that meet the physicochemical or structural definitions of 'superbase' include proton chelators like the aromaticproton sponge
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula CH(NMe) (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional b ...
s and the bispidines. Multicyclic polyamines
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. ...
, like DABCO might also be loosely included in this category.'' Phosphanes and carbodiphosphoranes are also strong organosuperbases. ''
Despite enormous proton affinity, the organosuperbases can exhibit low nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
.
Organometallic
 Organometallic compounds of electropositive metals are superbases, but they are generally strong nucleophiles. Examples include
Organometallic compounds of electropositive metals are superbases, but they are generally strong nucleophiles. Examples include organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
and organomagnesium (Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
) compounds. Another type of organometallic superbase has a reactive metal exchanged for a hydrogen on a heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecula ...
, such as oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
(unstabilized alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s) or nitrogen (metal amides such as lithium diisopropylamide).
The Schlosser base Schlosser's base (or Lochmann-Schlosser base) describes various superbasic mixtures of an alkyllithium compound and a potassium alkoxide. The reagent is named after Manfred Schlosser, although he uses the term ''LICKOR superbase'' (LIC denoting the ...
(or Lochmann-Schlosser base), the combination of ''n''-butyllithium and potassium ''tert''-butoxide, is commonly cited as a superbase. ''n''-Butyllithium and potassium ''tert''-butoxide form a mixed aggregate of greater reactivity than either component reagent.
Inorganic
Inorganic superbases are typicallysalt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
-like compounds with small, highly charged anions, e.g. lithium hydride, potassium hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold ...
, and sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in ...
. Such species are insoluble, but the surfaces of these materials are highly reactive and slurries are useful in synthesis.
Applications
Superbases are used inorganocatalysis
In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic co ...
.{{how, date=November 2022
See also
*Superacid
In chemistry, a superacid (according to the classical definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superaci ...
* Phosphazene
References